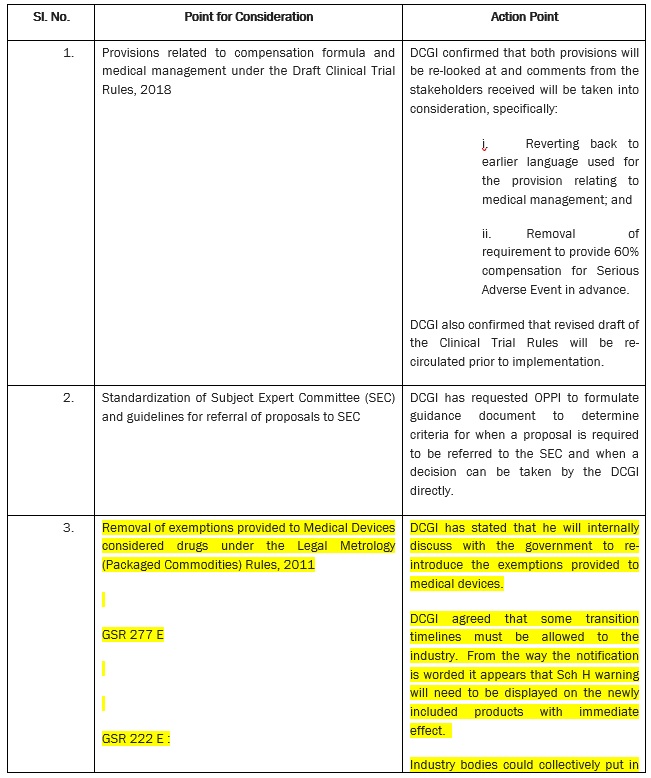
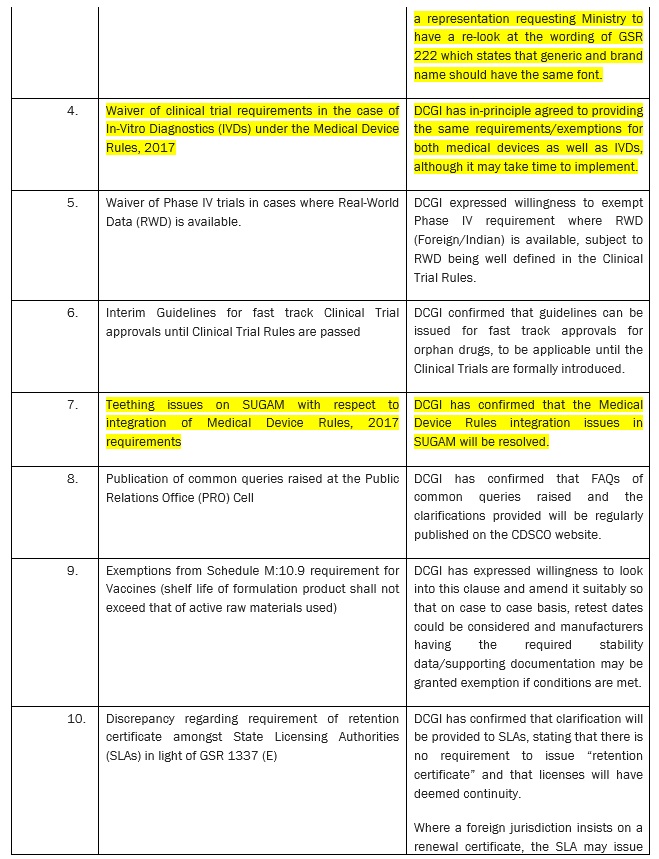
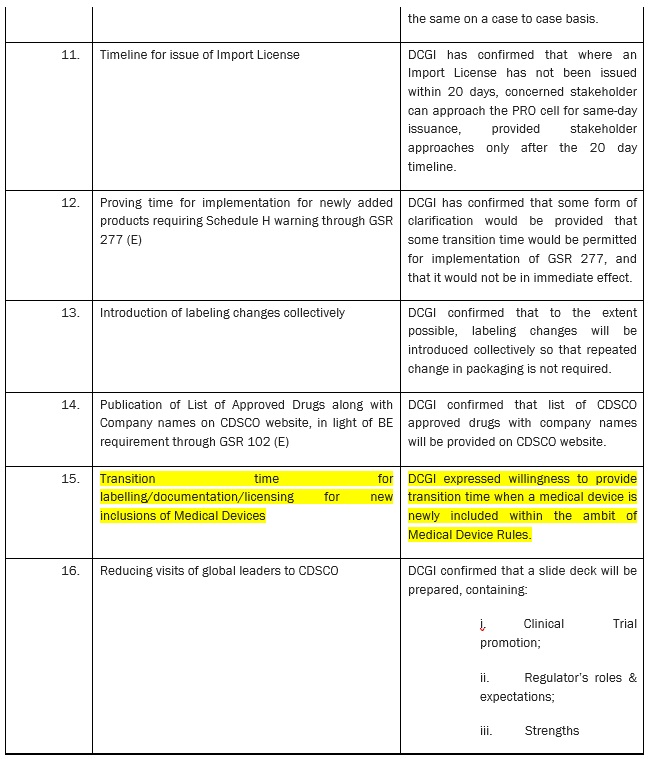
ACTION POINTS FROM DIALOGUE WITH DR. R. K. VATS, ADDITIONAL SECRETARY & DIRECTOR GENERAL, CGHS AND DR. ESWARA REDDY, DRUG CONTROLLER GENERAL (INDIA) (DCGI)
Summary on Devices
Medical devices should not be treated as drugs;
• Members are willing to subject themselves to a mandatory promotion code. DOP (Dept. of Pharmaceuticals) should ensure that there is level playing field in terms of ethical standards of promotion.
• Medical device companies have unique requirements. They should not be gove
rned by UCPMP as it has a deep pharma focus.
• It is difficult for Members to request HCPs to give a written request for samples. Such a request is highly impractical.
• Members should be allowed to leave brand reminders within pre-determined value cap. This is in-line with global practices.
Hon’ble Secretary took note of the Members concerns and commented the following:
• The UCPMP will continue to be a voluntary code until notified. There are challenges with regards to enforcement of UCPMP. Hence, it is not easy to make UCPMP mandatory.
• Medical device companies should prepare and present a draft code for medical devices promotion.
• Since UCPMP is a voluntary code, so compliance with such requirement is left upto the members.
• Members stressed that with the advance of science a lot of diseases which were incurable sometime ago are now curable. Further, the risk of irrational self-medication which arises as a result of advertisement of pharmaceuticals does not arise upon advertisement of medical devices. Therefore medical devices should be kept outside the purview of advertisement law.
• Members expressed concern that notified medical devices are required by law to limit their shelf life to five years whereas scientific evidence exists to increase shelf life of certain notified medical devices can be extended to 10 years.
Hon’ble Secretary noted the concerns of the Members and commented at his closing remarks that Separate consultation for UCPMP for medical device should take place
Participants
Pharma (Drugs)
Sanjeev Navangul, Managing Director, JNJ
Dr. Shailesh Ayyangar, Managing Direct, Sanofi
Yasmin Shenoy, Sr. Director, Regulatory, Sanofi
Bhavesh Kotak, Exec. Vice President, Medical, GSK
Sukanya Choudhury, Vice President-Regulatory Affairs, South Asia, GSK
Ransom DSouza, Vice President, Corporate Communications & Govt. Affairs
Dr. Suresh Menon, Novartis
Jeroze Dalal, Novartis
Vivek Kamath, Managing Director, MSD
Rahul Luthra, Exec. Dir. Regulatory Affairs
Dr. Chirag Trivedi, Sanofi (as President-ISCR)
Prabhat Jain, Head-Govt. Affairs, Abbott
Dr. Rashmi Hegde, Medical Director, Abbott
Suneela Thatte, Vice President, Quintiles
Shivprasad Laud, Company Secretary &Director, Roche Products
Seema Shimpi, Associate Director-Regulatory Affairs, Roche Products
Dr. Viraj Suvarna, Medical Director, Boehringer-Ingelheim
Dr. Ajay Sharma, OPPI
Dilip Shah, IPA
Dr. Thirumalai Velu, Director-Regulatory Affairs, Allergan
Ashok Bhattacharya, Managing Director, Takeda Pharma
Naveen Kumar Nagaraja, Associate Director-Regulatory Affairs, Takeda Pharma
Manali Agarwal, Head-Regulatory, Bayer
Amita Bhave, Director-Regulatory Affairs, Novartis
Rahul Sharma, Astra Zeneca
Annapurna Das, GSK
Dr. Shaswati Devsharma, Regulatory Head, Pfizer
Devices
Lubna Shaeikh, MTaI
Prabhat Jain, Head-Govt. Affairs, Abbott
Sushmita Chowdhury, Head-Regulatory Affair, B, Braun
Ravi Valia, Head-Govt. Affairs & Market Access, B Braun
Dr. Anish Desai, Medical Director, Johnson & Johnson
Rakesh Sahni, Regulatory Head, Johnsosn & Johnson
Aniruddha Patankar, Country Manager-Cardinal Health
Tanushree Ghatak, Manager-Regulatory Affairs, Cardinal Health
Kriti Sharma, Smith & Nephew
Devaleena Goswami, Smith & Nephew
Anay Shukla, Nishith Desai Associates
Darren Punnen, Nishith Desai Associates
LSW Lifescienceworld
www.lswlifescienceworld.com