
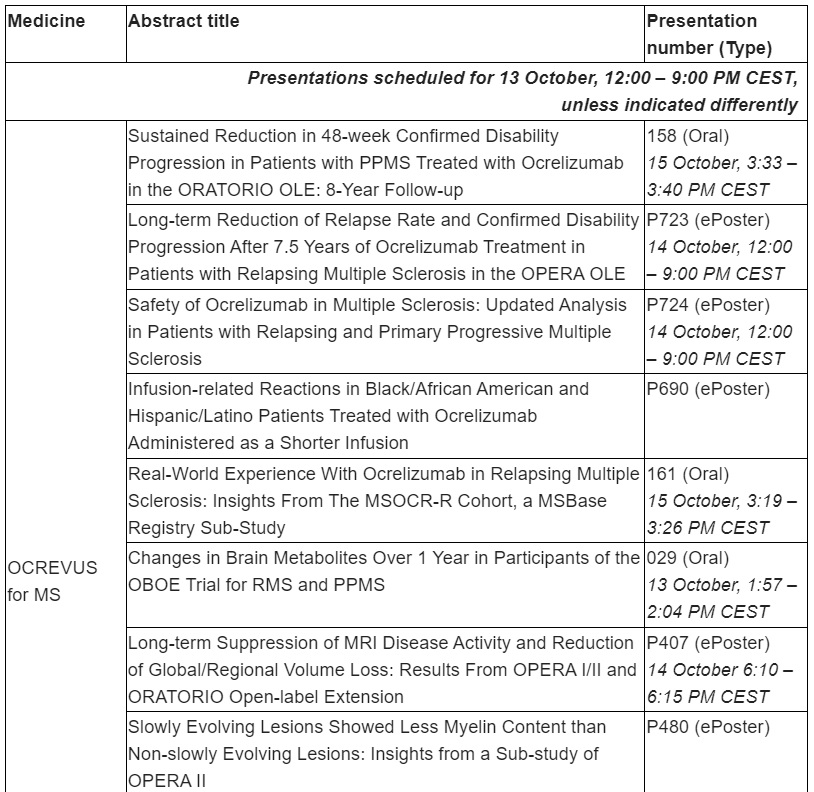
OCREVUS (ocrelizumab) data to show sustained reduction in disability progression through 8 years for primary progressive multiple sclerosis (PPMS) and 7.5 years for relapsing MS (RMS)
Long-term safety analysis of all clinical trials will reinforce the consistently favourable benefit-risk profile of OCREVUS
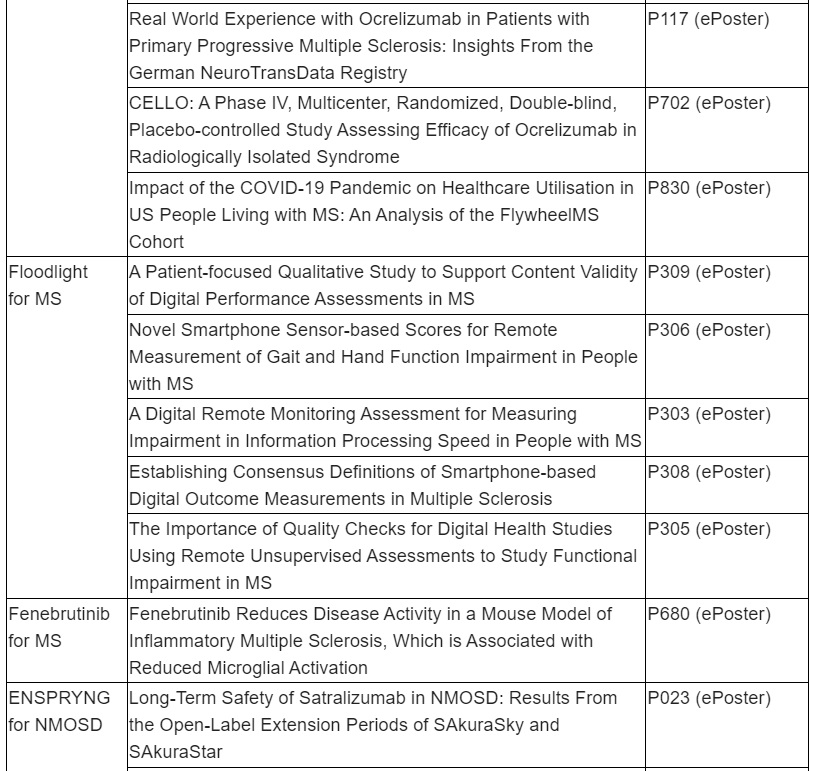
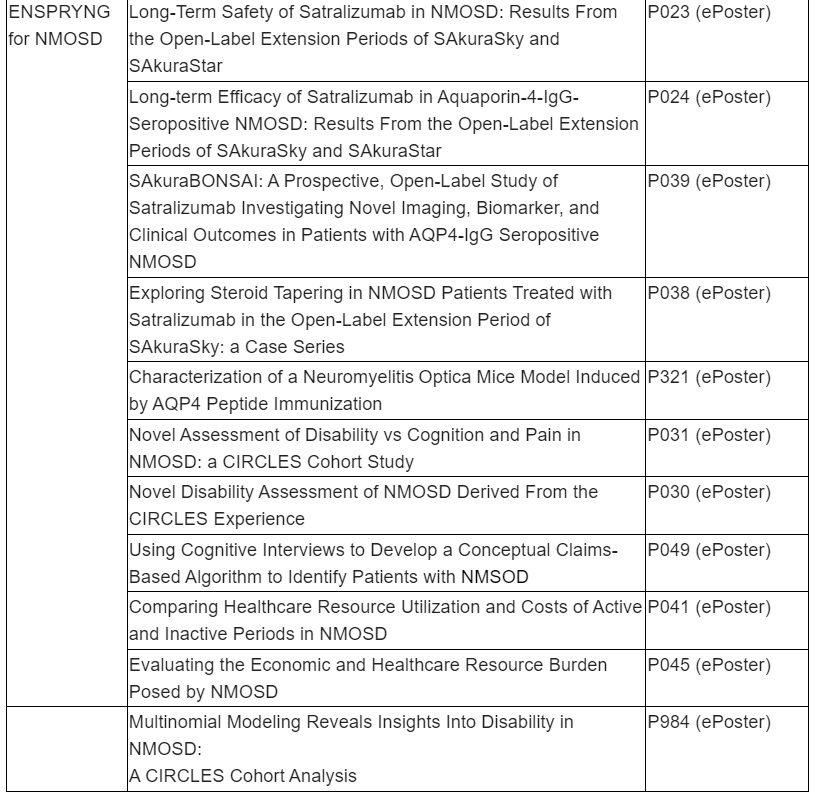
ENSPRYNG (satralizumab) data to show efficacy and safety sustained over four years of treatment for people living with neuromyelitis optica spectrum disorder (NMOSD)
Study design for SAkuraBONSAI, a new study on disease activity and progression in ENSPRYNG patients, who are treatment naïve or where prior rituximab (or biosimilar) treatment has failed, will be presented
Basel, 5 October 2021 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that new OCREVUS® (ocrelizumab) and ENSPRYNG® (satralizumab) data will be presented at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) from 13 - 15 October 2021. These data include 38 abstracts highlighting new longer-term efficacy and safety for both OCREVUS and ENSPRYNG, as well as our ongoing efforts to evaluate the impact of the COVID-19 pandemic for people living with MS. Additional data will show how a deeper scientific understanding of MS and NMOSD in diverse patient populations could help ensure access to treatment.
“The longer-term efficacy and safety data for both OCREVUS and ENSPRYNG reinforce the impact of these treatments - by significantly slowing disease progression in MS and by preventing debilitating relapses in NMOSD, respectively,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “We continue to see that early and ongoing treatment markedly improves outcomes, and we’ll continue to use scientific and real-world insights to improve our understanding and ways to support people living with these neurological disorders.”
Multiple sclerosis (MS)
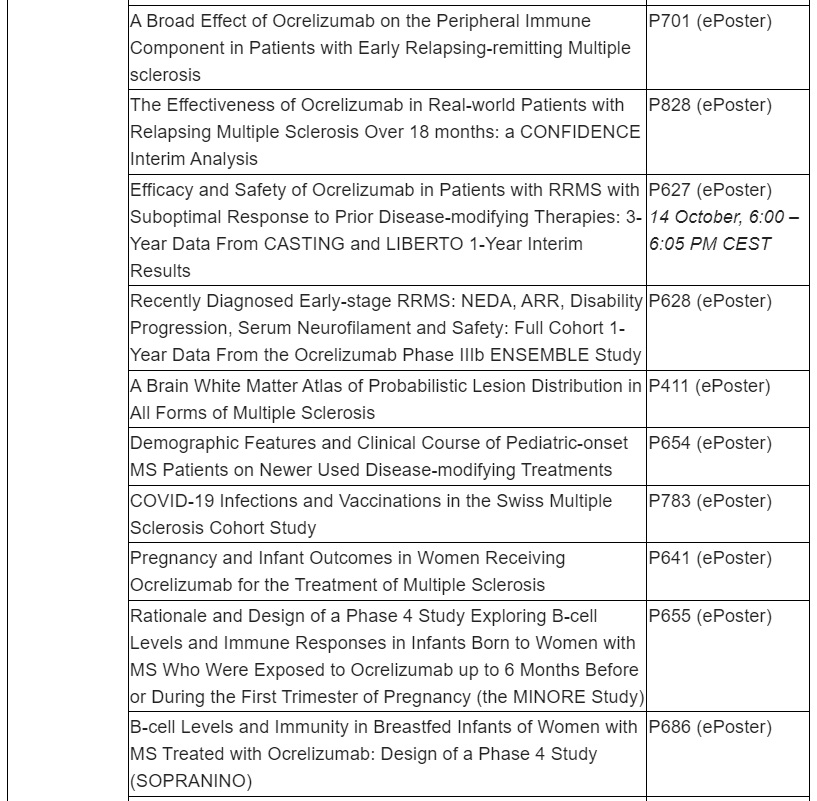
Roche will present 27 MS studies, including long-term data that show earlier treatment with OCREVUS continues to impact disability progression up to 8 years in people with primary progressive multiple sclerosis (PPMS) and up to 7.5 years in people with relapsing multiple sclerosis (RMS) in the Phase III open label extension studies. Additionally, updated long-term safety analysis of all clinical trials in patients with RMS and PPMS will reinforce the consistently favourable benefit-risk profile of OCREVUS.
Roche remains committed to addressing health disparities and we believe inclusive research can improve outcomes and derive insights that may address treatment barriers. A subgroup analysis of three studies (SaROD, CHORDS ENSEMBLE PLUS) in Black, African-American, Hispanic and Latino populations treated with a 2-hour OCREVUS infusion will be presented.
Neuromyelitis optica spectrum disorder (NMOSD)
New longer-term results from the SAkuraStar and SAkuraSky OLE studies for ENSPRYNG will show efficacy observed in the pivotal trials is sustained with high proportions of patients remaining free from relapse over four years of treatment. Similarly, safety data from the SAkuraStar and SAkuraSky OLE studies will show the favourable safety profile of ENSPRYNG is sustained with longer-term treatment. ENSPRYNG has been approved in 58 countries globally, including in the U.S. as the first and only subcutaneous treatment for adults with anti-aquaporin-4 antibody (AQP4-IgG) seropositive NMOSD. ENSPRYNG has also been approved for both adults and adolescents in the European Union, Japan, Canada and Switzerland.
Roche is dedicated to increasing scientific understanding of NMOSD and improving care for all people living with the condition. The study design will be presented for SAkuraBONSAI, a prospective, open-label study of ENSPRYNG to generate data to further the understanding of the disease activity and mechanism of action of ENSPRYNG in patients living with AQP4-IgG seropositive NMOSD who are treatment naïve or where prior rituximab (or biosimilar) treatment has failed. Other presentations will examine the development of new tools and techniques to identify patients with NMOSD and assess disability better.
Follow Roche on Twitter via @Roche and keep up to date with ECTRIMS 2021 news and updates by using the hashtag #ECTRIMS2021.
About OCREVUS® (ocrelizumab)
OCREVUS is the first and only therapy approved for both RMS (including RRMS and active, or relapsing, secondary progressive MS [SPMS], in addition to clinically isolated syndrome [CIS] in the U.S.) and PPMS. OCREVUS is a humanised monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to myelin (nerve cell insulation and support) and axonal (nerve cell) damage. This nerve cell damage can lead to disability in people with MS. Based on preclinical studies, OCREVUS binds to CD20 cell surface proteins expressed on certain B cells, but not on stem cells or plasma cells, suggesting that important functions of the immune system may be preserved. OCREVUS is administered by intravenous infusion every six months. The initial dose is given as two 300 mg infusions given two weeks apart. Subsequent doses are given as single 600 mg infusions.
About ENSPRYNG® (satralizumab)
ENSPRYNG, which was designed by Chugai, a member of the Roche Group, is a humanised monoclonal antibody that targets interleukin-6 (IL-6) receptor activity. The cytokine IL-6 is believed to be a key driver in NMOSD disease processes, triggering the inflammation cascade and leading to damage and disability. ENSPRYNG was designed using novel recycling antibody technology. When compared to conventional antibodies, ENSPRYNG’s recycling antibody technology enables the medicine to remain in the bloodstream for a longer period of time and bind repeatedly to its target (the IL-6 receptor) - maximally sustaining IL-6 suppression in a chronic disease like NMOSD and enabling subcutaneous dosing every four weeks.
Positive Phase III results for ENSPRYNG, as both monotherapy and in combination with baseline immunosuppressive therapy, suggest that IL-6 inhibition is an effective therapeutic approach for NMOSD. The Phase III clinical development programme for ENSPRYNG includes two studies: SAkuraStar and SAkuraSky.
ENSPRYNG is currently approved in 58 countries, including the United States, Canada, Japan, South Korea and the European Union.
ENSPRYNG has been designated as an orphan drug in the United States, Europe, Japan and Russia. In addition, it was granted Breakthrough Therapy Designation for the treatment of NMOSD by the FDA in December 2018, which is given to treatments that may demonstrate substantial improvement over other available options.
About Roche in neuroscience
Neuroscience is a major focus of research and development at Roche. Our goal is to pursue groundbreaking science to develop new treatments that help improve the lives of people with chronic and potentially devastating diseases.
Roche is investigating more than a dozen medicines for neurological disorders, including multiple sclerosis, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Duchenne muscular dystrophy and autism spectrum disorder. Together with our partners, we are committed to pushing the boundaries of scientific understanding to solve some of the most difficult challenges in neuroscience today.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan