
First data to be presented from the phase II coopERA Breast Cancer study evaluating neoadjuvant giredestrant treatment for oestrogen receptor (ER)-positive, HER2-negative breast cancer
New data from the phase III IMpower010 study provide further insights into the role of Tecentriq® (atezolizumab) in early-stage non-small cell lung cancer (NSCLC)
Genomic data from the phase II CUPISCO study in Cancer of Unknown Primary (CUP), an uncommon type of cancer with high unmet need, could support diagnostics and more personalised treatment plans
Basel, 07 September 2021 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that new oncology data will be presented at the European Society for Medical Oncology (ESMO) Congress, which will be held 16-21 September, 2021. With one of the broadest oncology pipelines and portfolios in the industry, Roche presentations include late-breaking abstracts featuring data in early-stage breast cancer and early-stage lung cancer, and a presentation on cancer of unknown primary (CUP), which has been selected for inclusion in the ESMO Press Programme.
“Our data at ESMO 2021 show how we continue to pursue potentially transformative advances in breast and lung cancer, particularly in earlier stage settings where the chance for cure is highest,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “Furthermore our efforts in personalised healthcare, exemplified by our insights in CUP, will help address the needs of many patients who are diagnosed at a late stage
Breast Cancer Highlights
First phase II data will be presented from an interim analysis of giredestrant, a next-generation oral selective oestrogen receptor degrader (SERD), in neoadjuvant, ER-positive, HER2-negative early breast cancer. Over 70% of breast cancer cases are hormone receptor (HR)-positive, and there is a need for more effective and tolerable treatments, since up to 30% of patients develop resistance to standard of care treatments and in the adjuvant setting half of patients stop treatment due to the toll of side effects. Our efforts in this area represent a step towards making the treatment of patients with HR-positive breast cancer more effective and less debilitating in order to reduce the burden of treatment. This study further showcases Roche’s commitment to identifying meaningful treatment options for patients with breast cancer through a comprehensive development programme that includes early and late-stage HR-positive, HER2 positive and triple-negative forms of the disease.
Lung Cancer Highlights
New results from the phase III IMpower010 study, to be presented during Presidential Symposium Three, highlight patterns of relapse and subsequent therapy following treatment with Tecentriq® (atezolizumab) versus standard of care in early lung cancer, and help to define how Tecentriq fits into the adjuvant treatment pathway. These findings are particularly important as real-world data, which will also be presented at ESMO, will provide detail on the proportion of people with early NSCLC in the United States who do not receive adjuvant treatment despite guidelines recommending that they do so. These real world data also highlight survival outcomes in this setting and reinforce the need for more effective adjuvant therapy in NSCLC, as half of all people with early-stage lung cancer today still experience disease recurrence following surgery.
The phase III IMpower010 study showed adjuvant Tecentriq improved disease-free survival in PD-L1-positive early-stage lung cancer, compared with best supportive care - a first in cancer immunotherapy. Based on the outcomes from this study, the United States Food and Drug Administration recently granted Priority Review under the Real-Time Oncology Review pilot programme to Tecentriq as an adjuvant treatment for certain people with early NSCLC, and is expected to make a decision later this year.
Innovation in Personalised Healthcare
Latest results from a preliminary descriptive molecular analysis based on the CUPISCO study, which will be featured in the ESMO Press Programme, shed further light on the genomic profiles of patients with poor-prognosis CUP. These results highlight the importance of comprehensive genomic profiling for patients with CUP and identify therapeutically relevant genomic alterations in a significant proportion of patients, which may help to inform a more personalised treatment plan. In CUP, doctors cannot identify the location of the original (primary) tumour and can only find metastases. This causes practical problems since traditional treatment approaches rely on the site of origin being known, and as such, most patients are treated with nonspecific chemotherapy. Unfortunately, prognosis remains poor and the median survival following diagnosis is just six to 12 months, reinforcing the need for improved diagnosis and treatment.
Real world data from the United States examining the use of Foundation Medicine’s comprehensive genomic testing prior to first-line therapy in patients with metastatic colorectal cancer (mCRC) will also be presented and underpin Roche’s efforts to drive more widespread and earlier testing for patients with cancer.
Ahead of ESMO, Roche will also be hosting a virtual Personalised Healthcare in Oncology Symposium for healthcare professionals on Thursday 9 September, 13:00 – 14:30 CEST. In this symposium, different healthcare system experts from across the world will discuss how new and emerging solutions in personalised healthcare can help to improve patient outcomes, and ultimately their quality of life.
LinkedIn Live event - COVID-19 and Cancer: Reshaping Patient Care in the Context of the Pandemic Today and Tomorrow
Roche will also be hosting a live roundtable discussion on Wednesday 15 September 2021, 16:00 CEST that will focus on the global efforts to overcome the impact of the COVID-19 pandemic on cancer outcomes. The discussion, featuring experts from medical societies, academia and patient groups, will focus on what lessons have been learned so far, and how stakeholders can forge new paths and partnerships to find and deliver solutions for patients and society.
Roche Oncology Newsroom
Roche’s ESMO Oncology Newsroom will be available from Tuesday 7 September for journalists to access exclusive materials sharing insights into Roche’s latest data, vision and strategy to pursue and advance scientific progress in order to improve the lives of people living with cancer. To access the newsroom, please register here.
Keep up to date with ESMO news and updates by using the hashtag #ESMO21 and follow Roche on Twitter via @Roche and LinkedIn.
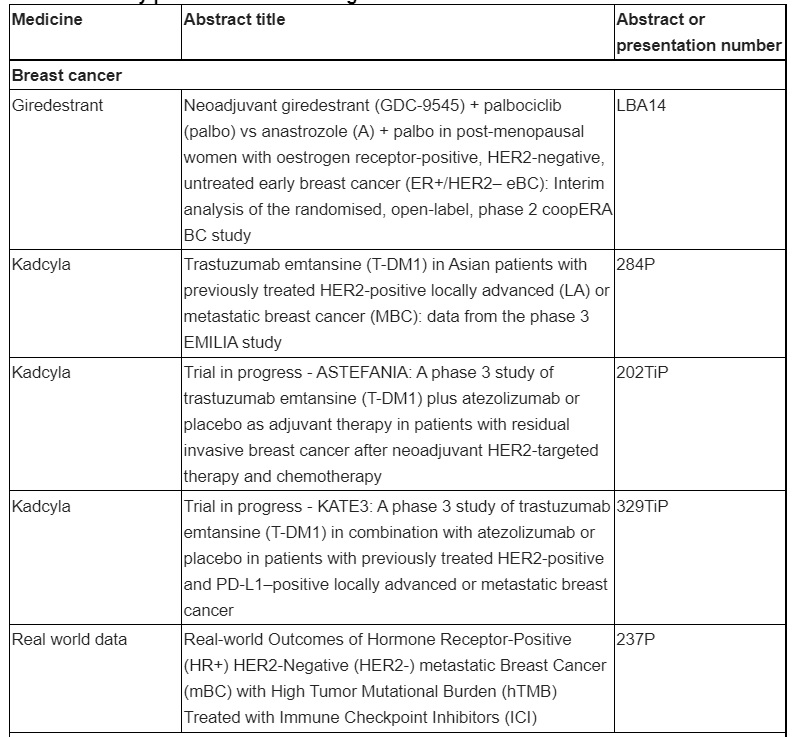
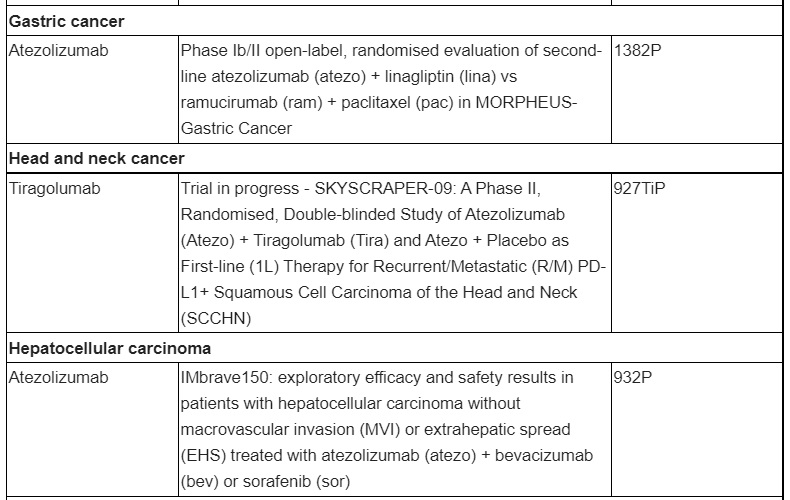
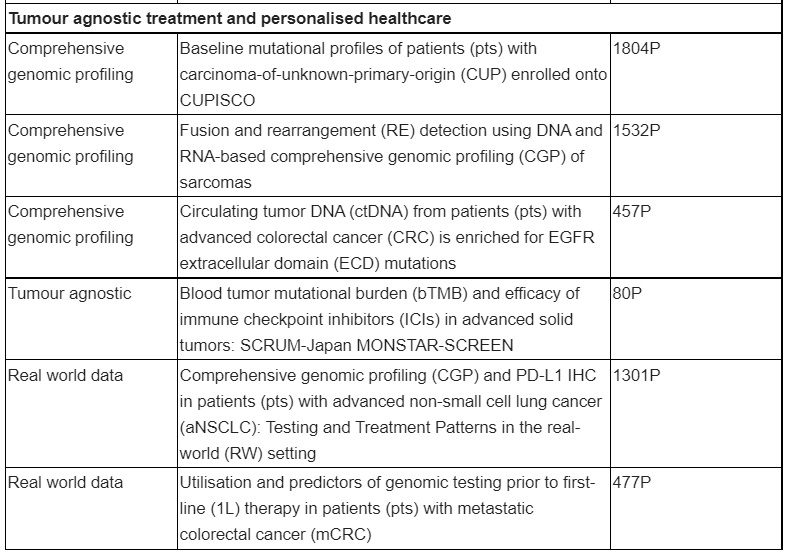
Overview of key presentations featuring Roche medicines

About Roche in Oncology
Roche has been working to transform cancer care for more than 50 years, bringing the first specifically designed anti-cancer chemotherapy drug, fluorouracil, to patients in 1962. Roche’s commitment to developing innovative medicines and diagnostics for cancers remains steadfast. The Roche Group’s portfolio of innovative cancer medicines includes: Alecensa®(alectinib); Avastin®(bevacizumab); Cotellic®(cobimetinib); Erivedge®(vismodegib); Gavreto®(pralsetinib); Gazyva®/Gazyvaro®(obinutuzumab); Herceptin®(trastuzumab); Kadcyla®(trastuzumab emtansine); MabThera®/Rituxan®(rituximab); Perjeta®(pertuzumab); Polivy®(polatuzumab vedotin); Tarceva®(erlotinib); Rozlytrek®(entrectinib); Tecentriq®(atezolizumab); Venclexta®/Venclyxto®(venetoclax) in collaboration with AbbVie; Xeloda®(capecitabine); Zelboraf®(vemurafenib). Furthermore, the Roche Group has a robust investigational oncology pipeline focusing on new therapeutic targets and novel combination strategies. For more information on Roche’s approach to cancer.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan