
Data for EVRYSDI reinforce safety profile and efficacy in a broad spinal muscular atrophy (SMA) population, following recent EU approval
Data for ENSPRYNG in neuromyelitis optica spectrum disorder (NMOSD) build on safety profile and efficacy following recent CHMP opinion, including in adults with concomitant autoimmune diseases (CAIDs)
OCREVUS data continue to show consistent benefit on slowing disease progression in relapsing MS (RMS) and primary progressive MS (PPMS)
Additional presentations in Alzheimer’s disease (AD), Huntington’s disease (HD) and Parkinson’s disease (PD) continue to contribute to understanding of these complex neurological disorders
Basel, 15 June 2021 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that data across its growing neuroscience portfolio will be presented at the 7th Congress of the European Academy of Neurology (EAN) Annual Meeting being held virtually 19-22 June, 2021. These new and encore data demonstrate Roche’s commitment to advancing the clinical understanding of a broad range of neurological disorders with the goal of meeting the needs of people living with both the rarest and most common conditions.“
Our data at EAN and recent European regulatory milestones for EVRYSDI and ENSPRYNG reflect our continued commitment to discovering and developing breakthrough medicines for challenging neurological conditions.” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “We are honoured to work with our partners and the broader community to accelerate progress across our neuroscience portfolio with the goal of transforming the lives of many people living with neurological disorders.”
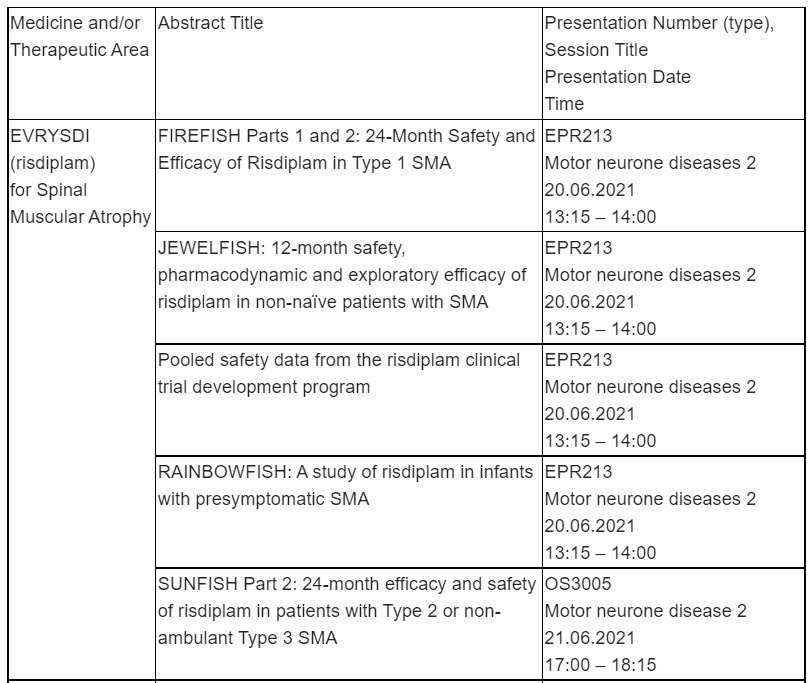
Spinal Muscular Atrophy (SMA)
Roche will present updated data from across the extensive EVRYSDI™ (risdiplam) clinical development programme, designed to represent a broad spectrum of people living with SMA, including those who have previously been treated with another SMA medication.
Among the five abstracts featured, new data includes 12-month safety, pharmacodynamic and interim exploratory efficacy data from the JEWELFISH study of EVRYSDI in people previously treated with SMA-targeting therapies across a broad range of ages (1–60 years), SMA types (1–3) and SMN2 copy number (1-5).
Data from SUNFISH Part 2 supporting the safety profile and efficacy of EVRYSDI in people aged 2-25 years with SMA Types 2 or non-ambulant Type 3 after two years of treatment will also be shared, as well as updated 2-year safety and efficacy data from the pivotal FIREFISH Parts 1 and 2 showing continued improvements in survival and motor milestones in infants aged one to seven months with Type 1 SMA.
In addition, preliminary safety and efficacy data from the RAINBOWFISH study of EVRYSDI treatment in pre-symptomatic babies from birth to six weeks will be presented, along with longer-term safety data from a separate pooled analysis of the FIREFISH, SUNFISH, JEWELFISH and RAINBOWFISH trials.
EVRYSDI, the first and only at home SMA treatment, is now approved in 42 countries, including the U.S. and EU. More than 3,000 patients have been treated with EVRYSDI in clinical trials, compassionate use and real-world settings.
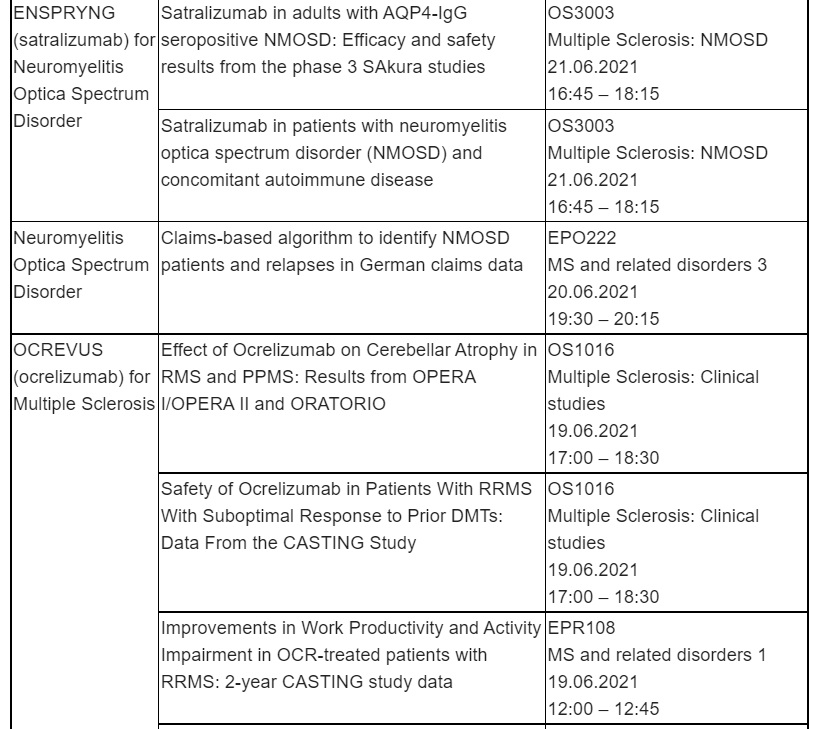
Neuromyelitis Optica Spectrum Disorder (NMOSD)
Roche will share three sets of data on people living with NMOSD, including an analysis of the Phase III SAkuraStar and SAkuraSky clinical trials which show ENSPRYNG® (satralizumab) significantly reduced risk of relapse vs placebo in adults with AQP4-IgG seropositive (AQP4-IgG+) NMOSD, with a favourable safety profile.
A separate pooled data analysis from the SAkura studies will also be presented, reinforcing favourable safety and efficacy of ENSPRYNG in people with NMOSD, including those with concomitant autoimmune diseases (CAIDs). Results from a new claims-based algorithm to identify people with NMOSD and NMOSD relapses in German claims data will also be shared.
ENSPRYNG is currently approved in 24 countries and has received a positive CHMP opinion as the first and only subcutaneous treatment for adults and adolescents with AQP4-IgG seropositive NMOSD in the EU, enabling home dosing by a person with NMOSD or their care partner.
Multiple Sclerosis (MS)
Four OCREVUS® (ocrelizumab) presentations will be shared, including an analysis of the Phase IIIb CASTING study in people with relapsing-remitting MS (RRMS) who had a suboptimal response to one or two prior disease-modifying therapies (DMTs). OCREVUS improved work productivity over two years, which correlated with reduced symptom burden and improvement in the physical and psychological impacts of MS.
Data from a separate subgroup analysis of the CASTING study will also be presented, continuing to show a favourable benefit-risk profile for OCREVUS and comparable safety outcomes regardless of age, type, or number of prior DMTs.
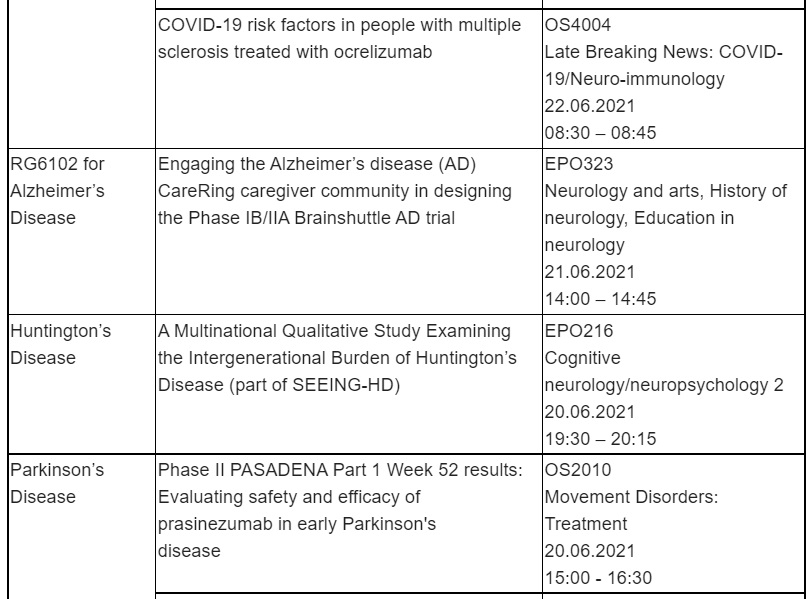
In addition, data from the OPERA and ORATORIO Phase III studies and their open-label extensions will be shared. A pooled analysis of the OPERA I and II studies, showed that OCREVUS reduced cerebellar atrophy in relapsing MS (RMS), compared with interferon beta-1a (IFN). An analysis of the open-label extension studies demonstrated that individuals initially treated with OCREVUS maintained lower cerebellar volume loss relative to baseline in both RMS and primary progressive MS (PPMS) during the open-label extension periods vs. those initially treated with comparators. OCREVUS is the first and only therapy approved for both RMS and PPMS with twice-yearly dosing, with over 200,000 patients treated globally.
Finally, a late-breaking oral abstract on risk factors for developing symptomatic or serious COVID-19 in patients treated with OCREVUS will be presented.
Alzheimer’s Disease (AD)
Roche will present an overview of the CareRing initiative, which engaged AD caregivers to input and provide guidance on trial design and protocol for the Phase IB/IIA Brain Shuttle AD trial, evaluating RG6102 in people with prodromal or mild-to-moderate AD.
RG6102 is a bispecific 2+1 monoclonal antibody that combines investigational gantenerumab with Roche’s Brain Shuttle technology. It is designed to be transported across the blood-brain barrier by engaging the transferrin receptor in order to achieve superior target engagement in the brain.
Huntington’s Disease (HD)
The protocol for a multinational qualitative study sponsored by Roche examining the burden of HD from a multidimensional, generational perspective (SEEING-HD) will be presented. Findings from this study will make an important contribution to advancing evidence-based care to improve the lives of individuals and families affected by HD.
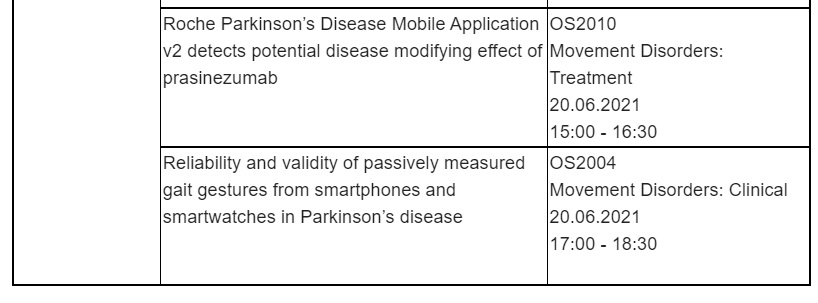
Parkinson’s Disease (PD)
Roche will share three presentations including further results from the Phase II PASADENA study evaluating the safety and efficacy of prasinezumab in early PD. In the PASADENA study, prasinezumab was the first anti-alpha-synuclein antibody to show evidence of slowing clinical decline of PD motor signs and demonstrated a favourable safety profile. These findings warrant further investigations in the recently initiated PADOVA study, studying the effect of prasinezumab in people with early Parkinson's disease receiving symptomatic therapy. In addition, data from two studies on digital health monitoring in PD will also be presented.
The full range of data from Roche’s clinical development programme in neuroscience being presented at EAN include:
About EVRYSDI™ (risdiplam)
EVRYSDI is a survival of motor neuron 2 (SMN2) splicing modifier designed to treat SMA by increasing production of the survival of motor neuron (SMN) protein. SMN protein is found throughout the body and is critical for maintaining healthy motor neurons and movement. EVRYSDI is administered daily at home in liquid form by mouth or by feeding tube.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved EVRYSDI for the treatment of SMA in adults and children 2 months of age and older. EVRYSDI was granted PRIME designation by the EMA in 2018 and Orphan Drug Designation by FDA and EMA in 2017 and 2019, respectively. At this time, EVRYSDI has been approved in seven countries and submitted in 57, including the EU 27 and Norway and Iceland.
About ENSPRYNG® (satralizumab)
ENSPRYNG, which was designed by Chugai, a member of the Roche Group, is a humanised monoclonal antibody that targets interleukin-6 (IL-6) receptor activity. The cytokine IL-6 is believed to be a key driver in NMOSD, triggering the inflammation cascade and leading to damage and disability. ENSPRYNG was designed using novel recycling antibody technology which, compared to conventional technology, allows for longer duration of the antibody and subcutaneous dosing every four weeks.
Positive Phase III results for ENSPRYNG, as both monotherapy and in combination with baseline immunosuppressive therapy, suggest that IL-6 inhibition is an effective therapeutic approach for NMOSD. The Phase III clinical development programme for ENSPRYNG included two studies: SAkuraStar and SAkuraSky.
ENSPRYNG is currently approved in 24 countries, including the United States, Canada, Japan, China and Switzerland.
ENSPRYNG has been designated as an orphan drug in the U.S., Europe and Japan. In addition, it was granted Breakthrough Therapy Designation for the treatment of NMOSD by the FDA in December 2018.
About OCREVUS® (ocrelizumab)
OCREVUS is the first and only therapy approved for both RMS (including clinically isolated syndrome, RRMS and active, or relapsing, SPMS) and PPMS, with dosing every six months. OCREVUS is a humanised monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to myelin (nerve cell insulation and support) and axonal (nerve cell) damage. This nerve cell damage can lead to disability in people with MS. Based on preclinical studies, OCREVUS binds to CD20 cell surface proteins expressed on certain B cells, but not on stem cells or plasma cells, suggesting that important functions of the immune system may be preserved.
OCREVUS is administered by intravenous infusion every six months. The initial dose is given as two 300 mg infusions given two weeks apart. Subsequent doses are given as single 600 mg infusions.
About Roche in Neuroscience
Neuroscience is a major focus of research and development at Roche. Our goal is to pursue groundbreaking science to develop new treatments that help improve the lives of people with chronic and potentially devastating diseases.
Roche is investigating more than a dozen medicines for neurological disorders, including spinal muscular atrophy, multiple sclerosis, neuromyelitis optica spectrum disorder, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Duchenne muscular dystrophy and autism spectrum disorder. Together with our partners, we are committed to pushing the boundaries of scientific understanding to solve some of the most difficult challenges in neuroscience today.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan