
Tecentriq improved disease-free survival by more than one-third in people with PD-L1-positive resectable early-stage lung cancer, compared with best supportive care
First and only cancer immunotherapy to show positive Phase III results in the adjuvant lung cancer setting
New adjuvant treatment options are urgently needed in early lung cancer to help the approximately 50% of people who currently experience recurrence following surgery
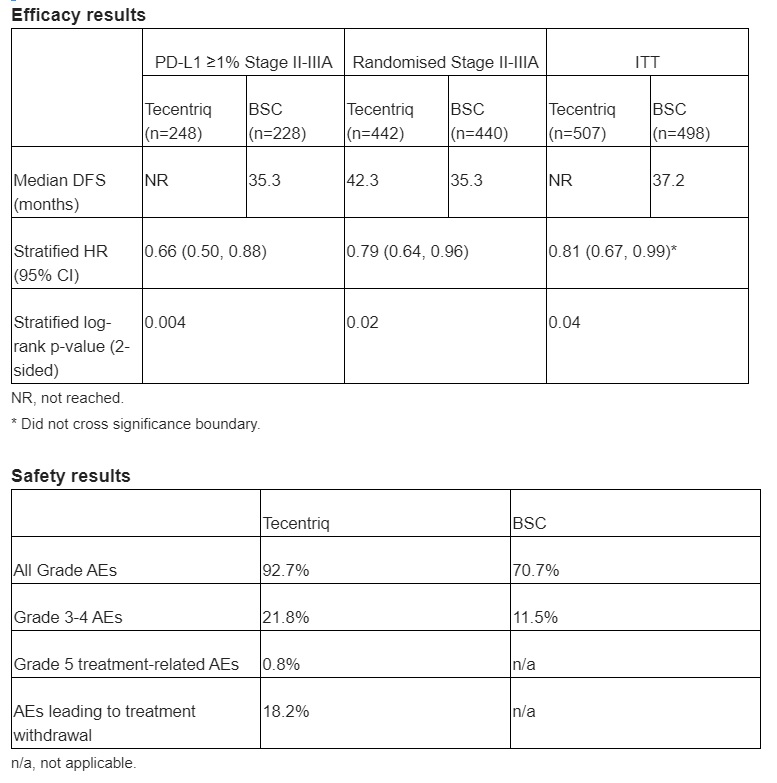
Basel, 20 May 2021 – Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced interim results from the Phase III IMpower010 study, showing for the first time that treatment with Tecentriq® (atezolizumab) following surgery and chemotherapy reduced the risk of disease recurrence or death (disease-free survival; DFS) by 34% (hazard ratio [HR]=0.66, 95% CI: 0.50–0.88) in people with Stage II-IIIA non-small cell lung cancer (NSCLC), whose tumours express PD-L1≥1%, compared with best supportive care (BSC). In this population, median DFS was not yet reached for Tecentriq compared with 35.3 months for BSC.
In the larger population of all randomised Stage II-IIIA study participants, Tecentriq reduced the risk of disease recurrence or death by 21% (HR=0.79, 95% CI: 0.64–0.96) after a median follow-up of 32.2 months.1 In this population, Tecentriq increased DFS by a median of seven months (42.3 months versus 35.3 months with BSC).1 Safety data for Tecentriq were consistent with its known safety profile and no new safety signals were identified. The full results of IMpower010 will be presented in the lung cancer oral abstract session (Abstract #8500) on Sunday 6 June (08:00–11:00 EDT) at the 2021 ASCO Annual Meeting.
“These landmark Phase III data demonstrate for the first time that cancer immunotherapy can bring a clinically meaningful improvement to certain people with early lung cancer in the adjuvant setting,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “These results lay the groundwork for a new approach to the treatment of early-stage lung cancer and bring us closer to our goal of providing an effective and tailored treatment option for every person diagnosed with this disease.”
The goal of adjuvant therapy is to lower the risk of recurrence and provide the best opportunity for a cure. Still, about half of all patients with Stage I-III NSCLC eventually develop disease recurrence following curative-intent treatment.2 Adjuvant platinum-based chemotherapy is the current standard of care for patients with completely resected early-stage NSCLC (Stage IB-IIIA) who are at a high-risk of disease recurrence or relapse. This treatment provides a modest 4–5% improvement in five-year survival compared with observation.3
Follow-up will continue with planned analyses of DFS in the overall intent-to-treat (ITT) population, including Stage IB patients, which at the time of analysis did not cross the threshold, and overall survival (OS) data, which were immature at the time of interim analysis. In the overall randomised population of study participants, adverse events (AEs) occurred in 92.7% of people receiving Tecentriq, compared with 70.7% of those receiving BSC. Grade 3 or 4 events occurred in 21.8% of people treated with Tecentriq compared with 11.5% in the BSC group; 0.8% of people in the Tecentriq group experienced a Grade 5 AE. As anticipated, the addition of up to one year of Tecentriq following chemotherapy led to a higher number of AEs compared with BSC.
Tecentriq has previously shown clinically meaningful benefit in various types of lung cancer, with five currently approved indications in markets around the world. It was the first approved cancer immunotherapy for front-line treatment of adults with extensive-stage small cell lung cancer (SCLC) in combination with carboplatin and etoposide (chemotherapy). Tecentriq also has four approved indications in NSCLC as either a single agent or in combination with targeted therapies and/or chemotherapies. Tecentriq is available in three dosing options, providing the flexibility to choose administration every two, three or four weeks.
Roche has an extensive development programme for Tecentriq, including multiple ongoing and planned Phase III studies across different settings in lung, genitourinary, skin, breast, gastrointestinal, gynaecological, and head and neck cancers. This includes studies evaluating Tecentriq both alone and in combination with other medicines, as well as studies in metastatic, adjuvant and neoadjuvant settings across various tumour types.
About the IMpower010 study
IMpower010 is a Phase III, global, multicentre, open-label, randomised study evaluating the efficacy and safety of Tecentriq compared with BSC, in participants with Stage IB-IIIA NSCLC (UICC 7th edition), following surgical resection and up to 4 cycles of adjuvant cisplatin-based chemotherapy. The study randomised 1,005 people with a ratio of 1:1 to receive either at most 16 cycles of Tecentriq or BSC. The primary endpoint is investigator-determined DFS in the PD-L1-positive Stage II-IIIA, all randomised Stage II-IIIA and ITT Stage IB-IIIA populations. Key secondary endpoints include OS in the overall study population, ITT Stage IB-IIIA NSCLC.
About NSCLC
Lung cancer is one of the leading causes of cancer death globally.4 Each year 1.8 million people die as a result of the disease; this translates into more than 4,900 deaths worldwide every day.4 Lung cancer can be broadly divided into two major types: NSCLC and SCLC. NSCLC is the most prevalent type, accounting for around 85% of all cases.5 NSCLC comprises non-squamous and squamous-cell lung cancer, the squamous form of which is characterised by flat cells covering the airway surface when viewed under a microscope.
About Tecentriq
Tecentriq is a monoclonal antibody designed to bind with a protein called Programmed Death Ligand-1 (PD-L1), which is expressed on tumour cells and tumour-infiltrating immune cells, blocking its interactions with both PD-1 and B7.1 receptors. By inhibiting PD-L1, Tecentriq may enable the activation of T-cells. Tecentriq is a cancer immunotherapy that has the potential to be used as a foundational combination partner with other immunotherapies, targeted medicines and various chemotherapies across a broad range of cancers. The development of Tecentriq and its clinical programme is based on our greater understanding of how the immune system interacts with tumours and how harnessing a person’s immune system combats cancer more effectively.
Tecentriq is approved in the US, EU and countries around the world, either alone or in combination with targeted therapies and/or chemotherapies in various forms of NSCLC, SCLC, certain types of metastatic urothelial cancer, in PD-L1-positive metastatic triple-negative breast cancer and for hepatocellular carcinoma. In the US, Tecentriq is also approved in combination with Cotellic® (cobimetinib) and Zelboraf® (vemurafenib) for the treatment of people with BRAF V600 mutation-positive advanced melanoma.
About Roche in cancer immunotherapy
Roche’s rigorous pursuit of groundbreaking science has contributed to major therapeutic and diagnostic advances in oncology over the last 50 years, and today, realising the full potential of cancer immunotherapy is a major area of focus. With over 20 molecules in development, Roche is investigating the potential benefits of immunotherapy alone, and in combination with chemotherapy, targeted therapies or other immunotherapies with the goal of providing each person with a treatment tailored to harness their own unique immune system to attack their cancer. Our scientific expertise, coupled with innovative pipeline and extensive partnerships, gives us the confidence to continue pursuing the vision of finding a cure for cancer by ensuring the right treatment for the right patient at the right time.
In addition to Roche’s approved PD-L1 checkpoint inhibitor, Tecentriq® (atezolizumab), Roche’s broad cancer immunotherapy pipeline includes other checkpoint inhibitors, such as tiragolumab, a novel cancer immunotherapy designed to bind to TIGIT, individualised neoantigen therapies and T-cell bispecific antibodies.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan