
Pivotal data on glofitamab, a potential first-in-class CD20xCD3 T-cell engaging bispecific antibody, in heavily pre-treated patients with aggressive lymphoma, will be presented as part of our industry-leading haematology portfolio
Further studies exploring broad genomic testing to support informed treatment decisions for patients and advance cancer care approaches will be presented
Basel, 24 May 2022 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that new data from clinical trials of 18 approved and investigational medicines across more than 20 cancer types will be presented at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, which will be held 3-7 June, 2022. Roche and its partners will present clinical studies across medicines, comprehensive genomic tests, and real-world data at this year’s meeting.
“At ASCO this year, progress from our portfolio, partnerships and collaborations showcase our commitment to advance innovation in cancer care,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “We’re especially pleased to present data from our broad haematology portfolio, including pivotal data for glofitamab, a potential first-in-class bispecific antibody that may improve the lives of people with heavily pre-treated aggressive lymphoma.”
Focusing on improving outcomes in non-Hodgkin lymphoma
New and updated data in non-Hodgkin lymphoma will be presented at ASCO. This includes pivotal data from the phase II NP30179 study evaluating glofitamab, an investigational CD20xCD3 T-cell engaging bispecific antibody, in heavily pre-treated patients with diffuse large B-cell lymphoma (DLBCL). DLBCL is an aggressive form of lymphoma, where as many as 40% of patients will relapse, at which point treatment options are limited and survival is shortened.1,2 Glofitamab is part of Roche’s broad bispecific antibody development programme, which may offer a new immunotherapy-based approach to tackle a range of blood cancers. It is being investigated in several clinical trials including the STARGLO phase III study, evaluating glofitamab in combination with gemcitabine and oxaliplatin (GemOx) versus MabThera®/Rituxan® (rituximab) in combination with GemOx in autologous stem-cell transplant ineligible relapsed or refractory DLBCL. In addition, key findings from an analysis of the Asia subpopulation from the pivotal phase III POLARIX study investigating Polivy® (polatuzumab vedotin) in combination with MabThera/Rituxan plus cyclophosphamide, doxorubicin and prednisone (R-CHP) in people with newly diagnosed DLBCL will be featured. Polivy plus R-CHP is the first treatment regimen to significantly improve outcomes in previously untreated DLBCL in more than 20 years, potentially transforming treatment for people with this disease.
Driving innovation in personalised cancer care
More than 20 new pieces of research from partnerships with Foundation Medicine will be presented, which continue to support innovation as well as progress in personalised cancer care. This includes new data from the phase II Profiler02 study,* which investigates the use of a comprehensive genomic profiling testing panel from Foundation Medicine, with the aim of informing possible treatment decisions for patients based on their tumour’s unique genomic information.
Data from the imCORE network
Additionally, three abstracts from the Immunotherapy Centers Of Research Excellence (imCORE) Network will be presented at ASCO: a phase I study investigating autogene cevumeran (an mRNA-based individualised neoantigen-specific immunotherapy [iNeST]***) in the adjuvant setting of pancreatic ductal adenocarcinoma;** a data mining study evaluating intermediate endpoints for survival in metastatic breast cancer in the real-world setting;** and a study identifying mechanisms of acquired resistance to immune checkpoint blockade.**
imCORE is an academic-industry network for scientific collaboration. Established by Roche and connecting experts from 26 leading institutions around the globe, imCORE is committed to advancing and accelerating cancer immunotherapy research. imCORE is an example of Roche’s dedication to collaborating with the global cancer community to further understand cancer biology and immunology, help inform the development of potential future treatment, and transform patients’ lives.
Roche’s data presented at ASCO will feature its efforts to drive innovation and commitment to health equity through delivery of pioneering medicines and personalised cancer care that together improve outcomes for every patient while reducing the cost to society, inclusive clinical trials that remove barriers to participation, partnerships that multiply our ability to address challenges in cancer care, and bringing innovation into earlier stages of disease to maximise a chance of cure.
Roche Oncology Newsroom
Roche’s Oncology Newsroom will be available to journalists from 24 May and feature exclusive materials providing insights into Roche’s vision, the latest data and perspectives on health inequities in cancer care. To access the Newsroom, please register here.
Overview of key presentations featuring Roche medicines

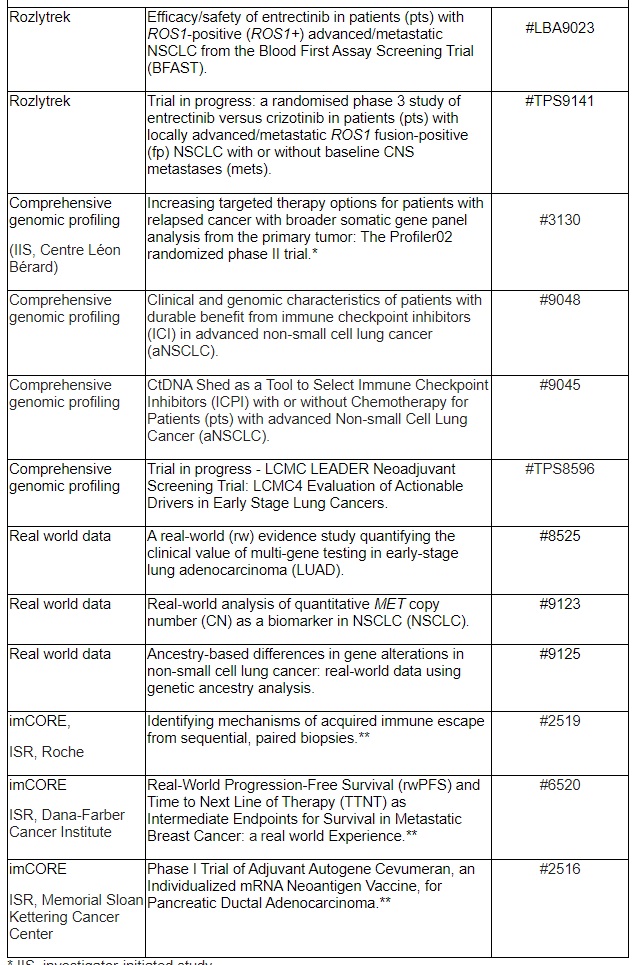
* IIS, investigator-initiated study
** ISR, institution-sponsored research
*** jointly developed by Roche and BioNTech
About Roche in Oncology
Roche has been working to transform cancer care for more than 50 years, bringing the first specifically designed anti-cancer chemotherapy drug, fluorouracil, to patients in 1962. Roche’s commitment to developing innovative medicines and diagnostics for cancers remains steadfast. The Roche Group’s portfolio of innovative cancer medicines includes: Alecensa® (alectinib); Avastin® (bevacizumab); Cotellic® (cobimetinib); Erivedge® (vismodegib); Gavreto® (pralsetinib); Gazyva®/Gazyvaro® (obinutuzumab); Herceptin® (trastuzumab); Kadcyla® (trastuzumab emtansine); MabThera®/Rituxan® (rituximab); Perjeta® (pertuzumab); Polivy® (polatuzumab vedotin); Tarceva® (erlotinib); Rozlytrek® (entrectinib); Tecentriq® (atezolizumab); Venclexta®/Venclyxto® (venetoclax) in collaboration with AbbVie; Xeloda® (capecitabine); Zelboraf® (vemurafenib). Furthermore, the Roche Group has a robust investigational oncology pipeline focusing on new therapeutic targets and novel combination strategies. For more information on Roche’s approach to cancer, visit www.roche.com.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan