
Phase III POLARIX trial showed Polivy plus R-CHP was the first treatment in two decades to significantly improve outcomes in newly diagnosed diffuse large B-cell lymphoma (DLBCL) versus the standard of care.
Pivotal data on mosunetuzumab, a potential first-in-class CD20xCD3 T-cell engaging bispecific antibody, showed high response rates in relapsed or refractory follicular lymphoma (FL).
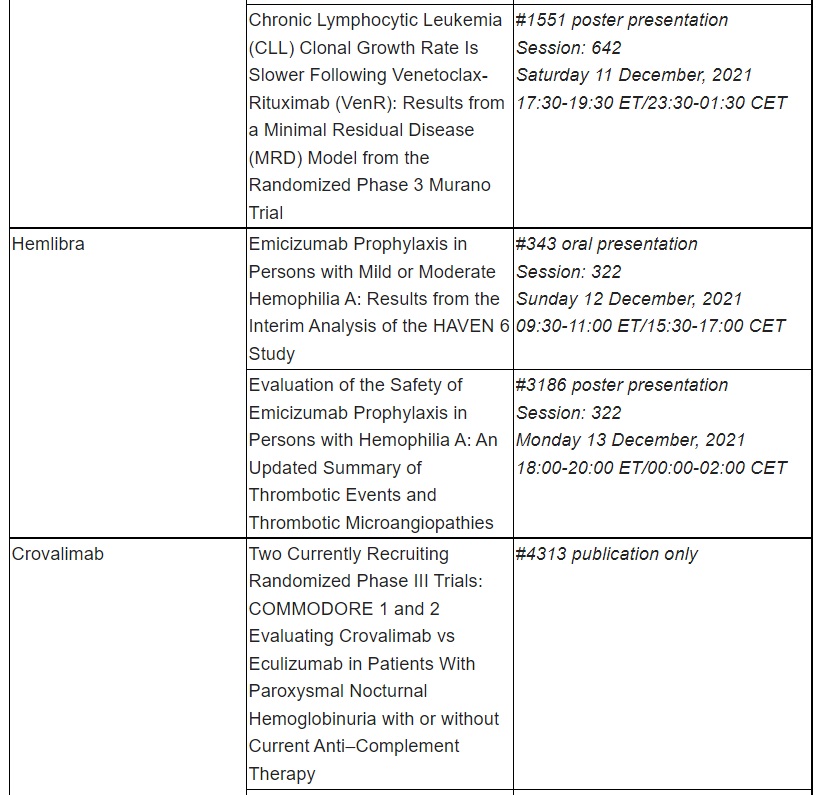
HAVEN 6 phase III interim data demonstrated Hemlibra’s favourable safety and efficacy profile in people with moderate or mild haemophilia A.
Basel, 23 November 2021 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that new data from its extensive haematology portfolio will be presented at the American Society of Hematology (ASH) Annual Meeting and Exposition from 11-14 December 2021. Roche molecules will be featured in more than 90 abstracts, including 17 oral presentations, showcasing new immunotherapies, unique treatment combinations, the application of novel endpoints, and fixed-duration regimens.
Results from three pivotal studies will be featured:
First presentation of efficacy and safety data from the phase III POLARIX study as a late-breaking abstract and in the ASH press programme. POLARIX met its primary endpoint of improving progression-free survival, showing Polivy® (polatuzumab vedotin) plus MabThera®/Rituxan® (rituximab), cyclophosphamide, doxorubicin, and prednisone (R-CHP) reduced the likelihood of disease worsening or death, versus the standard-of-care, MabThera/Rituxan plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), for people with newly diagnosed diffuse large B-cell lymphoma (DLBCL). The safety profile was comparable for Polivy plus R-CHP versus R-CHOP.1 POLARIX is being conducted in collaboration with The Lymphoma Study Association (LYSA) and The Lymphoma Academic Research Organisation (LYSARC).
Pivotal results from the phase I/II GO29781 study, presented for the first time and featured in the ASH press programme, showing mosunetuzumab, a CD20xCD3 T-cell engaging bispecific antibody immunotherapy, achieved high response rates with a manageable safety profile. These data suggest that it could be a new treatment option for people with relapsed or refractory follicular lymphoma (FL) who have received two or more prior therapies.2 FL is the most common indolent (slow growing) form of non-Hodgkin lymphoma, a type of blood cancer, which often returns after initial therapy.4
Interim data from the phase III HAVEN 6 study, which demonstrated the favourable safety and efficacy profile of Hemlibra® (emicizumab) in people with moderate or mild haemophilia A without factor VIII inhibitors.3 This patient population has historically not used prophylactic (preventative) treatments, likely due to delayed or missed diagnosis and a lack of treatments and treatment guidelines, meaning these patients have a significant unmet clinical need.5, 6
“For 20 years, we have remained committed to deepening our understanding of many benign and malignant blood disorders in order to better meet the urgent needs of patients with these diseases,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “Our data at ASH reinforce our conviction that following the science and developing versatile treatment approaches leads to improved outcomes for patients in increasingly meaningful ways.”
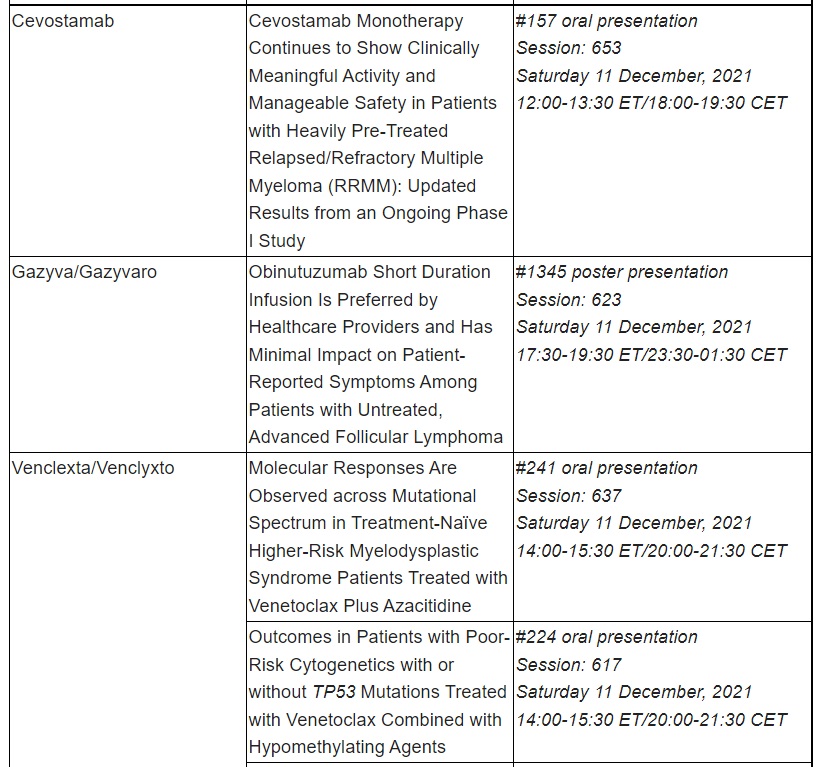
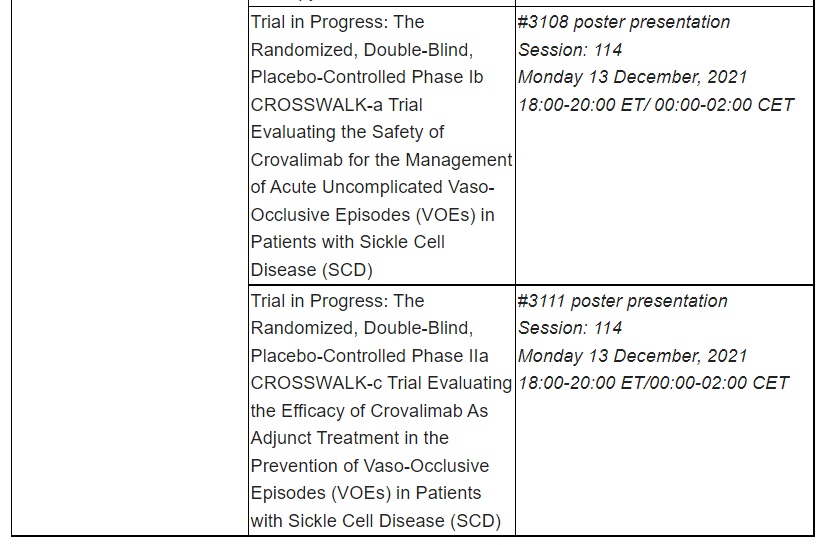
Data presented at ASH by Roche span numerous blood diseases, including lymphoma, leukaemia, multiple myeloma and haemophilia. Additional data to be presented include updated results for three T-cell engaging bispecific antibody immunotherapies: glofitamab and mosunetuzumab, targeting CD20 and CD3; and cevostamab, targeting FcRH5 and CD3.
Further information on the key abstracts featuring Roche medicines presented can be found in the table below.
Roche will be hosting a Live Media Event titled: ‘Redefining treatment standards to improve outcomes for patients with blood disorders’ on Wednesday 15 December, offering an exclusive opportunity to hear from experts about the pivotal data being presented. Register here to access the Roche Haematology Newsroom, available from Friday 26 November, where you’ll be able to attend the Live Media Event and find materials to further explain and provide context to Roche’s malignant and non-malignant data.
Follow Roche on Twitter via @Roche and LinkedIn and keep up to date with ASH Annual Meeting news and updates by using the hashtag #ASH21.



About Roche in haematology
Roche has been developing medicines for people with malignant and non-malignant blood diseases for over 20 years; our experience and knowledge in this therapeutic area runs deep. Today, we are investing more than ever in our effort to bring innovative treatment options to patients across a wide range of haematologic diseases. Our approved medicines include MabThera®/Rituxan® (rituximab), Gazyva®/Gazyvaro® (obinutuzumab), Polivy® (polatuzumab vedotin), Venclexta®/Venclyxto® (venetoclax) in collaboration with AbbVie, and Hemlibra® (emicizumab). Our pipeline of investigational haematology medicines includes T-cell engaging bispecific antibodies, glofitamab and mosunetuzumab, targeting both CD20 and CD3, and cevostamab, targeting both FcRH5 and CD3; Tecentriq® (atezolizumab), a monoclonal antibody designed to bind with PD-L1; and crovalimab, an anti-C5 antibody engineered to optimise complement inhibition. Our scientific expertise, combined with the breadth of our portfolio and pipeline, also provides a unique opportunity to develop combination regimens that aim to improve the lives of patients even further.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan