
Vabysmo data suggest rapid and robust drying of retinal fluid in patients with neovascular or ‘wet’ age-related macular degeneration and diabetic macular edema
Real-world studies of Vabysmo demonstrate ability to extend treatment intervals in the first four months while maintaining visual acuity
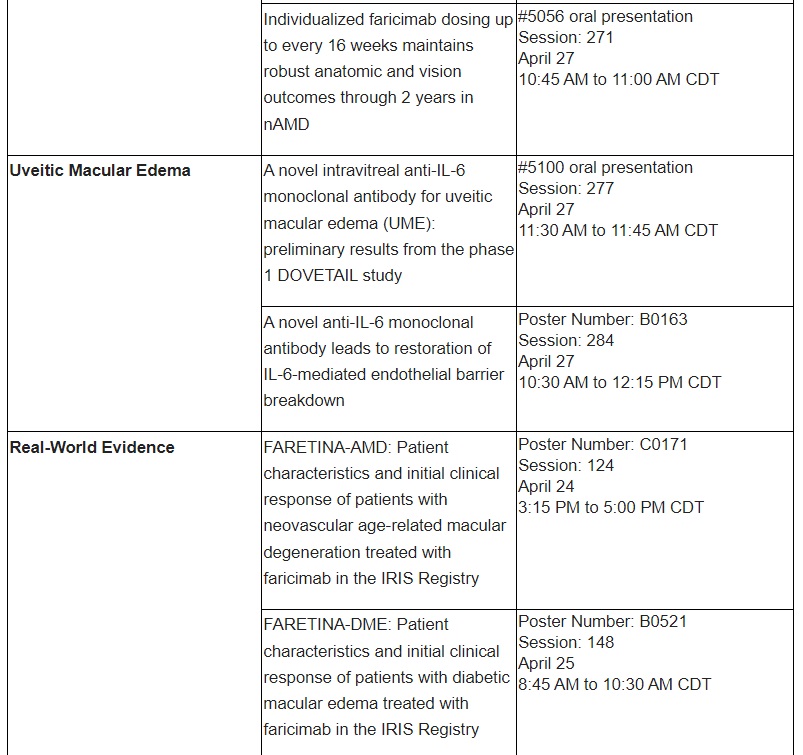
Clinical data on an investigational anti-interleukin-6 treatment in uveitic macular edema will be presented for the first time
Basel, 13 April 2023 - Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that new data for its approved and investigational medicines will be highlighted in 30 abstracts at the 2023 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, which will be held from 23-27 April 2023 in New Orleans, United States. The abstracts showcase the strength and breadth of Roche’s Ophthalmology portfolio, including post-hoc data from phase III Vabysmo® (faricimab) studies that support its benefit in drying retinal fluid in neovascular or ‘wet’ age-related macular degeneration (nAMD) and diabetic macular edema (DME).1-3 Real-world data on Vabysmo treatment patterns and outcomes will be presented, as well as approaches to personalised healthcare that include the use of artificial intelligence (AI) modelling to predict retinal disease progression.4-7 Additionally, phase I data for an investigational anti-interleukin-6 (IL-6) treatment in uveitic macular edema (UME), to be presented for the first time, suggest the monoclonal antibody may improve visual acuity in patients with UME.8
“The breadth of data we are presenting at ARVO demonstrates our sustained commitment to preserving vision for people with potentially blinding retinal conditions,” said Levi Garraway, M.D., Ph.D., Chief Medical Officer and Head of Global Product Development. “We are particularly encouraged by data indicating that Vabysmo may stabilise blood vessels and reduce fluid in the retina. Fluid control is essential for optimal central vision used for everyday activities such as reading and driving.”
The following data will be presented at ARVO 2023:
Vabysmo improves drying for people with nAMD and DME
A post-hoc analysis from the head-to-head dosing period of the phase III TENAYA and LUCERNE studies suggests Vabysmo results in greater drying of retinal fluid compared to aflibercept in people with nAMD. The data include change in central subfield thickness (CST), absence of subretinal and intraretinal fluid (SRF and IRF) and time to absence of SRF and IRF.
A post-hoc analysis from the head-to-head dosing period of the phase III YOSEMITE and RHINE studies also supports the positive impact of Vabysmo on macular blood vessel leakage compared to aflibercept in people with DME. Outcomes include macular leakage area and the proportion of patients with minimal to no macular leakage - two important markers of vascular stability. Another analysis from YOSEMITE and RHINE suggests Vabysmo reduces retinal fluid when compared to aflibercept in people with DME. The data include time to absence of DME (CST <325 µm) and time to absence of IRF.
Vabysmo extends dosing intervals early in the real world
Two Vabysmo real-world studies in nAMD and DME show patients extended their dosing intervals early in their treatment while maintaining or improving their vision. The majority of patients were able to extend their treatment intervals during the four initial doses. Treatment intervals were categorised as ‘extended’ if any interval was more than six weeks apart.
Investigational IL-6 monoclonal antibody may benefit people with UME
Phase I data on an IL-6 inhibitor that is in development for UME and other retinal conditions suggest that this investigational monoclonal antibody improves visual acuity and CST in patients with UME.
The IL-6 pathway plays an important role in the development and progression of UME by promoting blood vessel leakage and inflammation.9 UME is a complication of uveitis, a form of eye inflammation.10 This results in accumulation of fluid in the macula and can lead to significant visual impairment and vision loss.10 The estimated prevalence of uveitis is between 6 to 12 people per 10,000 globally, and approximately one-third of these people are impacted by UME.11
Roche recently launched two phase III trials in UME based on encouraging phase I safety and efficacy data. The first patients have been treated in the Meerkat (NCT05642312) and Sandcat (NCT05642325) studies, which are evaluating the safety and efficacy of the monoclonal antibody in people with UME.12,13 Roche is also studying the IL-6 inhibitor in DME.
AI and machine learning
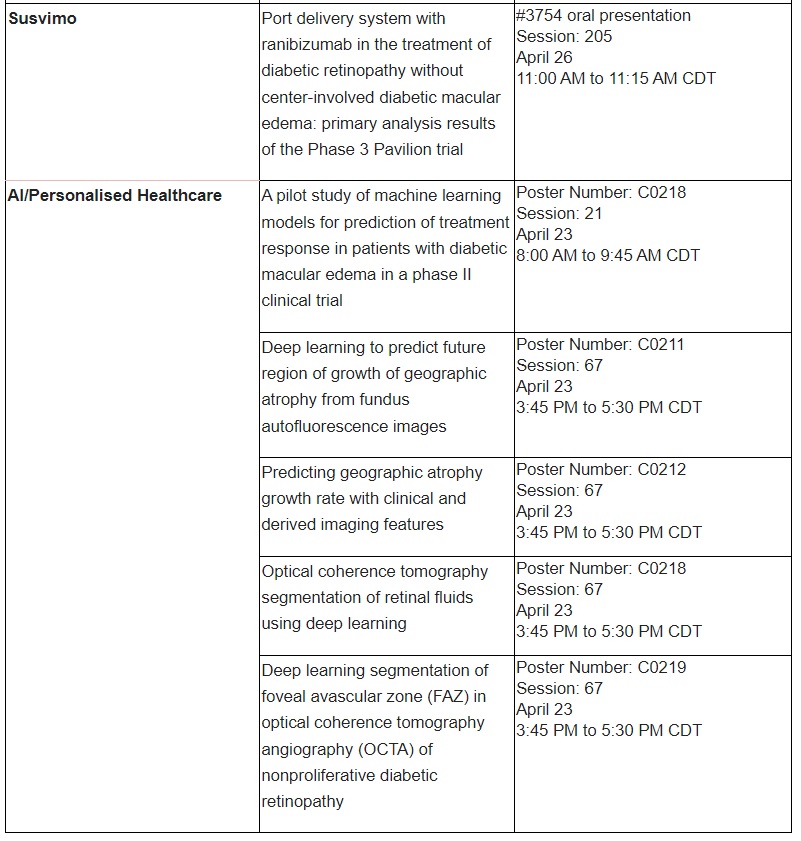
Roche will also present research related to the diagnosis and treatment of retinal conditions. These presentations include new research on the use of AI and machine learning to predict disease progression in geographic atrophy, a progressive and irreversible form of AMD; enable timely and accurate assessment of disease activity in nAMD or DME; predict treatment response in DME; and investigate new imaging biomarkers in diabetic retinopathy.
Further information on select Roche abstracts that will be presented at ARVO 2023 can be found in the table below.

About neovascular age-related macular degeneration
Age-related macular degeneration (AMD) is a condition that affects the part of the eye that provides sharp, central vision needed for activities like reading.18 Neovascular or ‘wet’ AMD (nAMD) is an advanced form of the disease that can cause rapid and severe vision loss if left untreated.19,20 It develops when new and abnormal blood vessels grow uncontrolled under the macula, causing swelling, bleeding and/or fibrosis.19 Worldwide, around 20 million people are living with nAMD – the leading cause of vision loss in people over the age of 60 – and the condition will affect even more people around the world as the global population ages.
About diabetic macular edema
Affecting around 21 million people globally, diabetic macular edema (DME) is a vision-threatening retinal condition associated with blindness and decreased quality of life when left untreated.23 DME occurs when damaged blood vessels leak into and cause swelling in the macula – the central area of the retina responsible for the sharp vision needed for reading and driving.24,25 The number of people with DME is expected to grow as the prevalence of diabetes increases.
About the Vabysmo® (faricimab) clinical development programme
Roche has a robust phase III clinical development programme for Vabysmo. The programme includes AVONELLE-X, an extension study of TENAYA and LUCERNE evaluating the long-term safety and tolerability of Vabysmo in neovascular or ‘wet’ age-related macular degeneration (nAMD), and Rhone-X, an extension study of YOSEMITE and RHINE evaluating the long-term safety and tolerability of Vabysmo in diabetic macular edema (DME).27,28 In addition, Roche is investigating the efficacy and safety of Vabysmo in people with macular edema following retinal vein occlusion in two phase III studies, BALATON and COMINO.29,30 Roche has also initiated several phase IV studies, including the Elevatum study of Vabysmo in underrepresented patient populations with DME, the SALWEEN study of Vabysmo in a subpopulation of nAMD highly prevalent in Asia, as well as the VOYAGER study, a global real-world data collection platform.31-33 Roche also supports several other independent studies to further understand retinal conditions with a high unmet need
About Vabysmo® (faricimab)
Vabysmo is the first bispecific antibody approved for the eye.34,35 It targets and inhibits two signalling pathways linked to a number of vision-threatening retinal conditions by neutralising angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).36,37 Ang-2 and VEGF-A contribute to vision loss by destabilising blood vessels, causing new leaky blood vessels to form and increasing inflammation.36,37 By blocking pathways involving Ang-2 and VEGF-A, Vabysmo is designed to stabilise blood vessels. Vabysmo is approved in 60 countries around the world, including the United States (U.S.), Japan, the United Kingdom and in the European Union for people living with neovascular or ‘wet’ age-related macular degeneration and diabetic macular edema. Review by other regulatory authorities is ongoing.
About Roche in ophthalmology
Roche is focused on saving people’s eyesight from the leading causes of vision loss through pioneering therapies. Through our innovation in the scientific discovery of new potential drug targets, personalised healthcare, molecular engineering, biomarkers and continuous drug delivery, we strive to design the right therapies for the right patients.
We have the broadest retina pipeline in ophthalmology, which is led by science and informed by insights from people with eye diseases. Our pipeline includes gene therapies and treatments for geographic atrophy and other vision-threatening diseases, including rare and inherited conditions.
Applying our extensive experience, we have already brought breakthrough ophthalmic treatments to people living with vision loss. Susvimo™ (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant is the first U.S. Food and Drug Administration-approved refillable eye implant for neovascular or ‘wet’ age-related macular degeneration that continuously delivers a customised formulation of ranibizumab over a period of months.40 Vabysmo® (faricimab) is the first bispecific antibody approved for the eye, which targets two signalling pathways that drive retinal conditions.34,37 Lucentis®* (ranibizumab injection) is the first treatment approved to improve vision in people with certain retinal conditions.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan