India’s pharmaceutical market for FY 2023-24 is valued at USD 50 billion with domestic consumption valued at USD 23.5 billion and export valued at USD 26.5 billion. India’s pharma industry is considered to be the world's third largest by volume and 14th in terms of value of production. With an extremely diversified product base covering generic drugs, bulk drugs, over-the-counter drugs, vaccines, biosimilars, and biologics, the Indian pharmaceutical industry has a strong presence at the global level. According to National Accounts Statistics 2024, published by the Ministry of Statistics and Programme Implementation, total output for industry i.e. Pharmaceuticals, medicinal and botanical products is Rs. 4,56,246 crores for FY 2022-23 at constant prices, of which value added is Rs. 1,75,583 crores. 9,25,811 number of persons are engaged in Pharmaceuticals, medicinal and botanical products industry during FY 2022-23.
Research & Development (R&D) and innovation in Pharma Sector is done by number of institutions and organizations under various scientific Ministries/Departments. The Department of Pharmaceuticals has set up seven National Institutes of Pharmaceutical Education & Research (NIPERs) as institutes of national importance, which besides imparting postgraduate and doctorate education, conduct high end research in various pharma specializations. Further, Department has framed a “National Policy on Research & Development and Innovation in Pharma-MedTech Sector in India” to encourage R&D in pharmaceuticals and medical devices and to create an ecosystem for innovation in the sector in order for India to become leader in drug discovery and innovative medical devices through incubating an entrepreneurial environment to build a robust ecosystem to ensure the holistic development of R&D and Innovation. The policy was notified on 18.08.2023.
The policy postulates three main areas of focus to achieve the objectives
To create a regulatory environment that facilitates innovation and research in product development, expanding the traditional regulatory objectives of safety and quality.
To incentivize private and public investment in innovation through a mix of fiscal and non-fiscal measures.
To build an enabling ecosystem designed to support innovation and cross-sectoral research as strong institutional foundation for sustainable growth in the sector.
The details of the policy document may be perused at the Gazette notification dated 18.08.2023 available in the public domain (https://pharmaceuticals.gov.in/policy). The Department has also framed a Scheme for Promotion of Research & Innovation in Pharma Sector (PRIP) with an outlay of Rs. 5000 crore for a period of 5 years i.e. 2023-24 to 2027-28, which was notified on 17.08.2023 with the objective of transforming Indian Pharma MediTech sector from cost-based to innovation-based by strengthening research infrastructure in the country. PRIP Scheme has two components:
Component A: Strengthening the Research Infrastructure by establishing Centres of Excellence (CoEs) in the seven existing National Institutes of Pharmaceutical Education & Research (NIPERs).
Component B: Promotion of Research in Pharma MediTech sector wherein financial assistance will be provided to the companies/projects for both in-house and academic R&D in six specified priority areas. The details of the scheme may be perused at the gazette notification dated 17.08.2023 available in the public domain (https://pharmaceuticals.gov.in/schemes)
Apart from the above, New Drugs and Clinical Trials Rules, 2019 have been notified on 19/03/2019, which contains various provisions for encouraging research and development and innovation of new drugs in the country. Key provisions are: -
• Disposal of clinical trial and new drug applications by way of approval or rejection or seeking further information within a period of 90 days.
• In case of application to conduct clinical trial of a new drug or investigational new drug as part of drug discovery, research and manufacture in India, the application is to be disposed of within a period of 30 days.
• In case of Clinical Trials application, if no communication is received from CDSCO within the prescribed timelines, the application will be deemed to have been approved.
• Provisions for accelerated/ expedited approval process in certain situation like unmet need, orphan drugs for rare diseases etc.
• Provisions for pre-submission and post submission meetings of the applicants with CDSCO for formal discussion and case specific regulatory pathway.
For export, the drug is required to be manufactured under manufacturing license as per provision of Drugs and Cosmetics Act and Rules thereunder. Further, the manufacturer is required to comply with the requirements of importing country.
As informed by the Department for Promotion of Industry & Internal Trade (DPIIT), Government has not taken any specific initiative especially for Pharmaceutical industry to strengthen their Intellectual property regime. However, several initiatives irrespective of the industry type has been undertaken to strengthen the Intellectual Property regime. The main initiatives undertaken in this regard are outlined below:-
1. Amendment of Rules i.e. Patents, Geographical Indication, The Trademark, Design and Copyright which are as under: -
Patents rules : Since 2014, Patents Rules have been amended several times to streamline and simplify filing and processing of patent applications, remove irregularities, redress procedural delays, and complexities in patent granting procedure, streamline the use of IT and digital technologies and provide certain benefits to the sectors that are critical for Indian economy. Some of the key changes made are viz: fee rebates of at least 80% for filing and processing of patent applications and maintenance of patents have been given to Startups, small entities (MSMEs) and educational institutes, facility of expedited examination has been given to Startups, small entities (MSMEs), applicants electing India an authority for international applications, female applicants, government institutions/ departments, electronic submission of documents by patent agents has been made mandatory and timelines have been streamlined, requirements for filing of a priority document and form 27 (statement regarding working of patents) have been streamlined, the time to submit request for examination has been reduced to 31 months from 48 months to fast-track the patent examination process and 10% reduction in the official fee for patent renewal is available if the fees for at least four years are paid in advance through electronic mode etc.
Start-Ups Intellectual Property Protection (SIPP) Scheme - “Start-Ups Intellectual Property Protection (SIPP)” scheme facilitates protection of Patents, Trademark and Designs by interested Startups, and all Indian innovators/ creators, educational institutes using the services of the Technology and Innovation Support Centers (TISCs) established in India. The Scheme was started in 2016 and has been further extended up to 31-03-2026. As on date, there are more than 2700 empanelled facilitators to help start-ups to file applications. The scheme has been revised recently and facilitation fees have been notably increased by at least 100%. The revised applicable fee structure is as under:
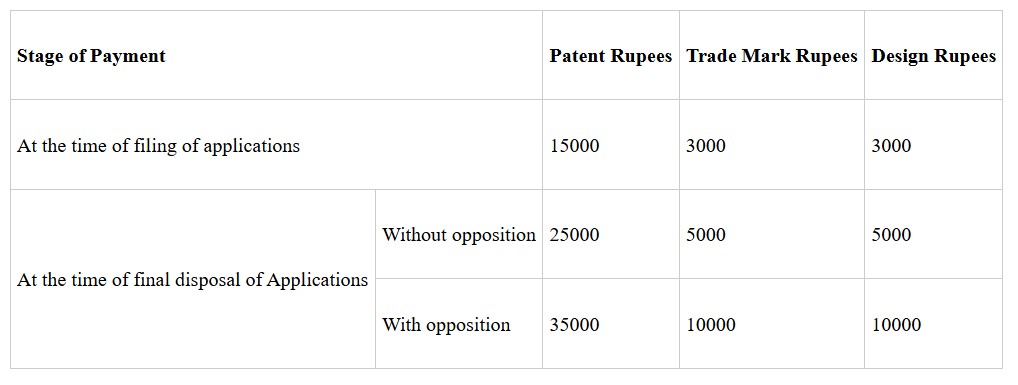
This information was given by the Union Minister of State for Chemicals and Fertilizers Smt Anupriya Patel in Rajya Sabha in a written reply to a question today.