
Late-breaking post-hoc data indicate Vabysmo leads to less fibrosis, which may negatively impact vision, than aflibercept in people with diabetic macular edema (DME)
Real-world data reinforce that first-line Vabysmo use improves outcomes and extends treatment intervals rapidly during the first four months for people with neovascular or ‘wet’ age-related macular degeneration (nAMD) and DME
Clinical data reiterate Vabysmo’s positive anatomical outcomes, including reduced blood vessel leakage in the macula and greater and faster retinal fluid control
Vabysmo is currently approved in over 70 countries to treat nAMD and DME, with more than one million doses distributed globally
Basel, 20 July - Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that data from its ophthalmology portfolio will be highlighted in 25 abstracts at the 2023 American Society of Retina Specialists (ASRS) Annual Meeting, which will be held from 28 July to 1 August in Seattle, United States. The data further advance the depth of clinical and real-world evidence supporting the use of Vabysmo® (faricimab), the first and only bispecific antibody for the eye, for the treatment of neovascular or ‘wet’ age-related macular degeneration (nAMD) and diabetic macular edema (DME).1-14
“The clinical and real-world data at ASRS reinforce the improvement in outcomes brought by Vabysmo in two leading causes of vision loss, particularly new analyses suggesting that Vabysmo is associated with less vision-impacting fibrosis than aflibercept,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “By improving disease control while offering a potentially less frequent treatment regimen, Vabysmo may help people spend less time managing their condition.”
Vabysmo is currently approved in over 70 countries to treat nAMD and DME, with public reimbursement in over 20 markets and more than one million doses distributed globally.15 Neovascular AMD and DME are two leading causes of vision loss worldwide, affecting more than 40 million people.16-19
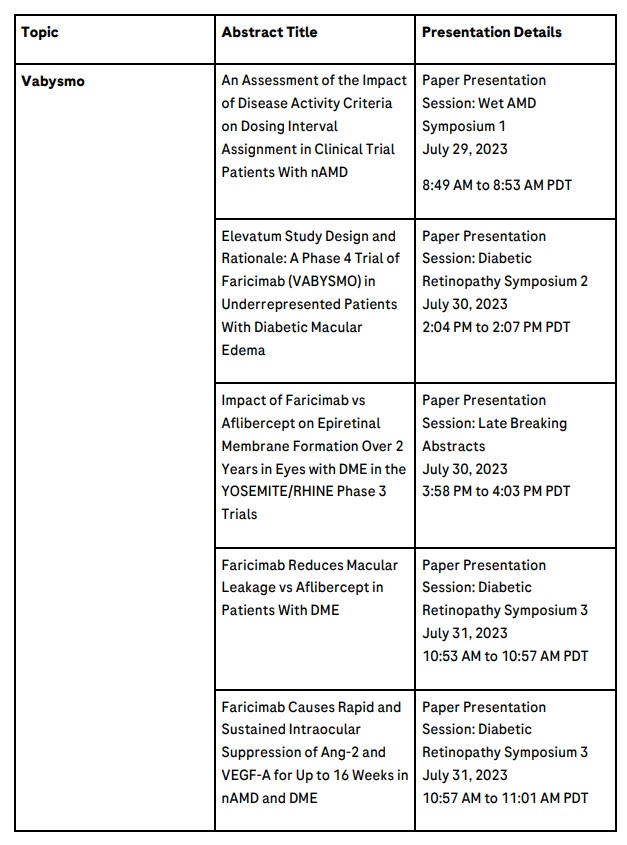
The following key data will be presented at ASRS 2023:
Late-breaker: Vabysmo’s effect on epiretinal membrane (ERM) formation in DME compared to aflibercept
Two-year post-hoc data from the YOSEMITE and RHINE phase III studies will be presented for the first time on ERM formation in DME patients, indicating Vabysmo leads to less retinal fibrosis than aflibercept.3 ERMs are fibrotic tissues on the surface of the retina, which may negatively impact the anatomy of the eye and compromise vision.20
Vabysmo drying and durability data
Data will be presented reiterating positive anatomical outcomes previously seen with Vabysmo treatment, including reduced blood vessel leakage in the macula, and greater and faster retinal fluid control.4,6,7 Blood vessel leakage can cause a build-up of fluid and swelling in the back of the eye, contributing to sight loss.21,22
Data will also further support how increased intervals between doses of Vabysmo to treat nAMD and DME, compared to aflibercept, do not compromise outcomes.
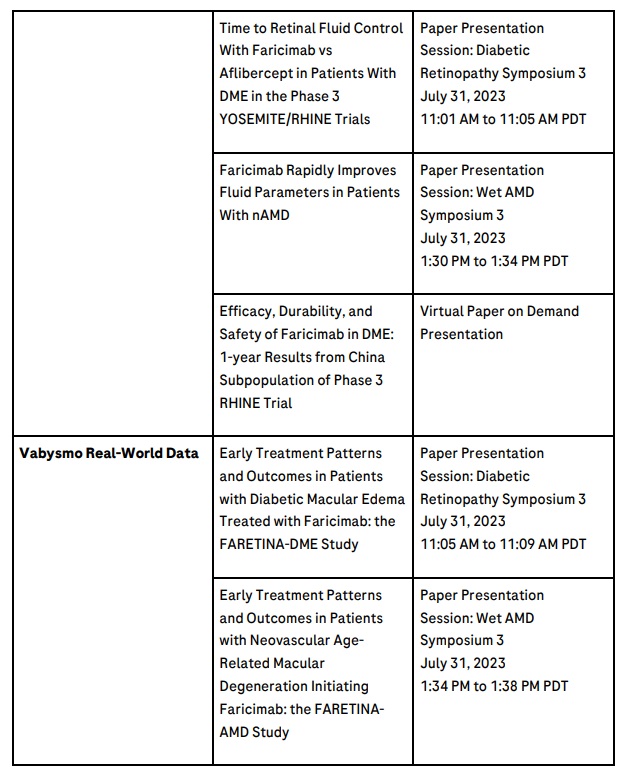
Vabysmo real-world data
Roche’s expanding programme of real-world studies for Vabysmo includes more than 8,500 participants in almost 30 countries.
Updates will be presented on real-world data from the FARETINA studies of Vabysmo in nAMD and DME looking at extended dosing intervals and impact on vision, including Vabysmo’s use as a first-line treatment.
Preliminary data on early outcomes and treatment patterns in the United Kingdom FARWIDE studies of Vabysmo in nAMD and DME will be shared for the first time.
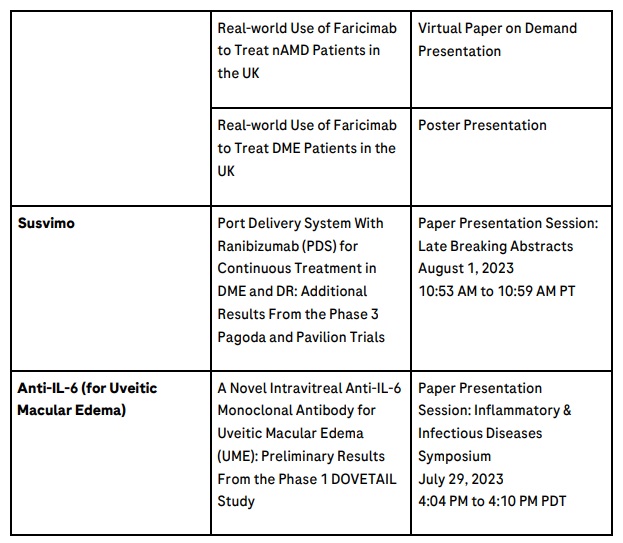
In addition, independent investigator studies of Vabysmo are expected to be presented. The TRUCKEE study, which focused on real-world outcomes in people with nAMD across 14 sites in the United States is scheduled for presentation on 31 July during the Wet AMD Symposium 3 (1:38 PM to 1:44 PM PDT).
Further information on select Roche abstracts that will be presented at ASRS 2023 can be found in the table below.
About neovascular age-related macular degeneration
Age-related macular degeneration (AMD) is a condition that affects the part of the eye that provides sharp, central vision needed for activities like reading.16 Neovascular or ‘wet’ AMD (nAMD) is an advanced form of the disease that can cause rapid and severe vision loss if left untreated.24,25 It develops when new and abnormal blood vessels grow uncontrolled under the macula, causing swelling, bleeding and/or fibrosis.24 Worldwide, around 20 million people are living with nAMD – the leading cause of vision loss in people over the age of 60 – and the condition will affect even more people around the world as the global population ages.
About diabetic macular edema
Affecting around 21 million people globally, diabetic macular edema (DME) is a vision-threatening retinal condition associated with blindness and decreased quality of life when left untreated.19 DME occurs when damaged blood vessels leak into and cause swelling in the macula – the central area of the retina responsible for the sharp vision needed for reading and driving.21,26 The number of people with DME is expected to grow as the prevalence of diabetes increases.
About the Vabysmo® (faricimab) clinical development programme
Roche has a robust phase III clinical development programme for Vabysmo® (faricimab). The programme includes AVONELLE-X, an extension study of TENAYA and LUCERNE evaluating the long-term safety and tolerability of Vabysmo in neovascular or ‘wet’ age-related macular degeneration (nAMD), and Rhone-X, an extension study of YOSEMITE and RHINE evaluating the long-term safety and tolerability of Vabysmo in diabetic macular edema (DME).28,29 In addition, Roche is investigating the efficacy and safety of Vabysmo in people with macular edema following retinal vein occlusion in two phase III studies, BALATON and COMINO.30,31 Roche has also initiated several phase IV studies, including the ELEVATUM study of Vabysmo in underrepresented patient populations with DME, the SALWEEN study of Vabysmo in a subpopulation of nAMD highly prevalent in Asia, as well as the VOYAGER study, a global real-world data collection platform.32-34 Roche also supports several other independent studies to further understand retinal conditions with a high unmet need.
About Vabysmo® (faricimab)
Vabysmo® (faricimab) is the first bispecific antibody approved for the eye.13,14 It targets and inhibits two signalling pathways linked to a number of vision-threatening retinal conditions by neutralising angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).35,36 Ang-2 and VEGF-A contribute to vision loss by destabilising blood vessels, causing new leaky blood vessels to form and increasing inflammation.35,36 By blocking pathways involving Ang-2 and VEGF-A, Vabysmo is designed to stabilise blood vessels. Vabysmo is approved in over 70 countries around the world, including the United States, Japan, the United Kingdom and in the European Union for people living with neovascular or ‘wet’ age-related macular degeneration and diabetic macular edema.13-15,37,38 Review by other regulatory authorities is ongoing.
About Roche in ophthalmology
Roche is focused on saving people’s eyesight from the leading causes of vision loss through pioneering therapies. Through our innovation in the scientific discovery of new potential drug targets, personalised healthcare, molecular engineering, biomarkers and continuous drug delivery, we strive to design the right therapies for the right patients.
We have the broadest retina pipeline in ophthalmology, which is led by science and informed by insights from people with eye diseases. Our pipeline includes gene therapies and treatments for geographic atrophy and other vision-threatening diseases, including rare and inherited conditions.
Applying our extensive experience, we have already brought breakthrough ophthalmic treatments to people living with vision loss. Susvimo™ (Port Delivery System with ranibizumab) 100 mg/mL for intravitreal use via ocular implant is the first U.S. Food and Drug Administration-approved refillable eye implant for neovascular or ‘wet’ age-related macular degeneration that continuously delivers a customised formulation of ranibizumab over a period of months.39 Vabysmo® (faricimab) is the first bispecific antibody approved for the eye, which targets and inhibits two signalling pathways linked to a number of vision-threatening retinal conditions by neutralising angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).13,14,35,36 Lucentis®* (ranibizumab injection) is the first treatment approved to improve vision in people with certain retinal conditions.40
*Lucentis® (ranibizumab injection) was developed by Genentech, a member of the Roche Group. Genentech retains commercial rights in the United States and Novartis has exclusive commercial rights for the rest of the world.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan