
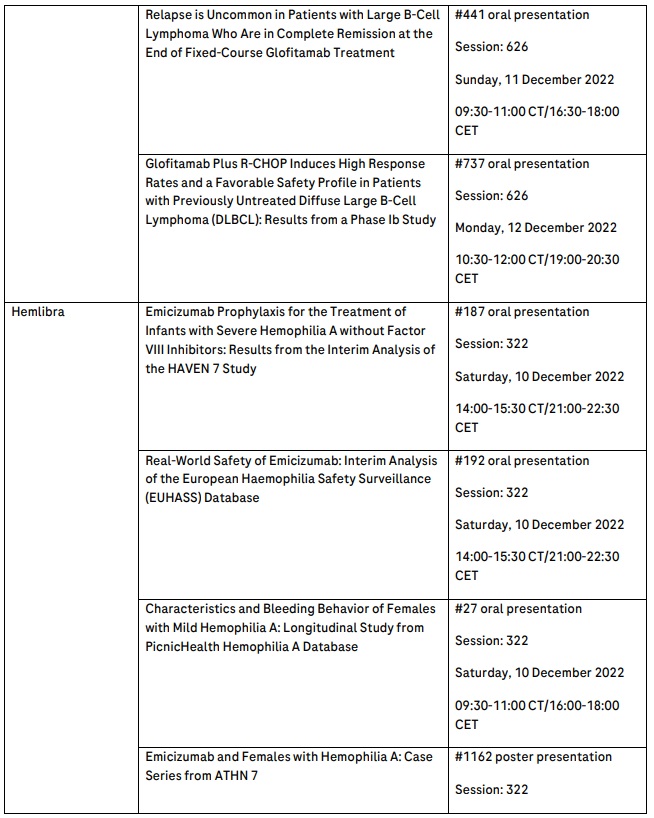
Interim data from phase III HAVEN 7 study reinforce Hemlibra’s efficacy and safety in infants with severe haemophilia A without factor VIII inhibitors1
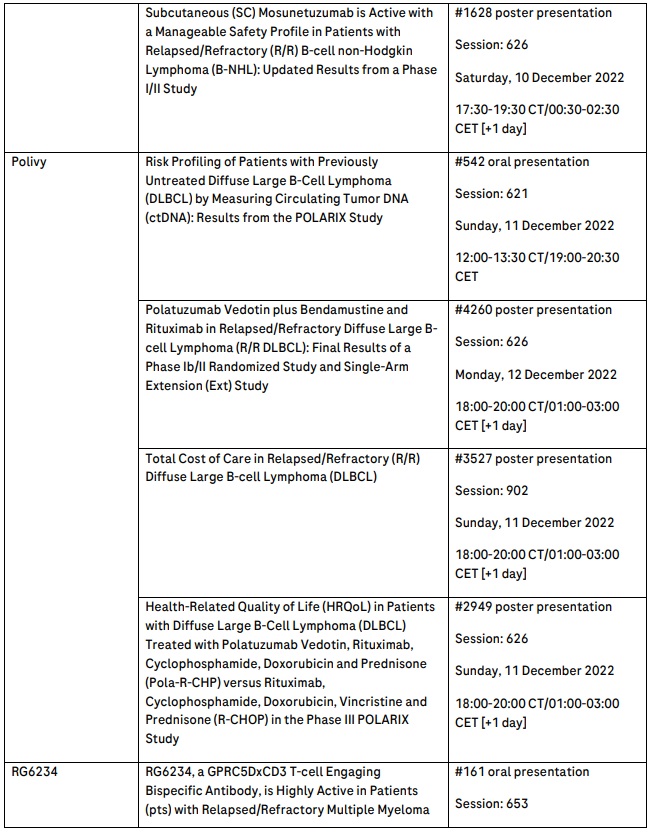
New and updated data support use of Polivy in diffuse large B-cell lymphoma, including its potential as a treatment option for previously untreated patients
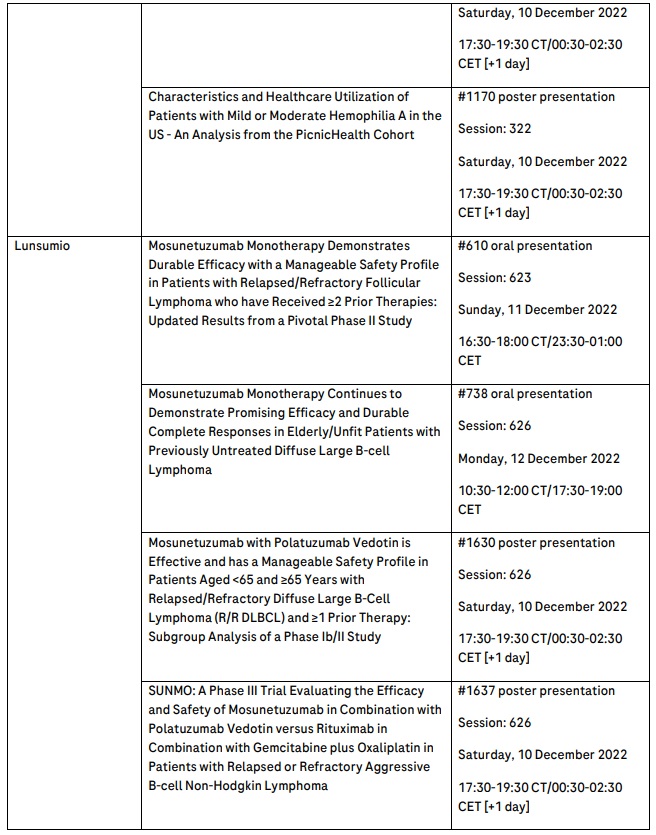
New and updated data for innovative CD20xCD3 T-cell engaging bispecific antibodies Lunsumio and glofitamab further enhance their potential as effective, off-the-shelf, fixed-duration treatment options for people with lymphoma
First phase III data for crovalimab show the co-primary efficacy endpoints were met, with subcutaneous injections achieving disease control in people with paroxysmal nocturnal haemoglobinuria as shown in COMMODORE 3 study in China
Basel, 3 November 2022 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that it will present new data from its industry-leading haematology portfolio at the 64th American Society of Hematology (ASH) Annual Meeting from 10-13 December 2022. The data to be presented span numerous blood diseases, including haemophilia A, paroxysmal nocturnal haemoglobinuria (PNH), and various types of blood cancers, including non-Hodgkin lymphoma (NHL) and multiple myeloma. Roche’s approved and investigational medicines will be featured in more than 50 abstracts, including more than 15 oral presentations.
“We continually strive to improve patient outcomes by exploring new treatment options across blood disorders, such as lymphomas and rare blood diseases, where unmet needs remain high,” said Levi Garraway, M.D., Ph.D., Chief Medical Officer and Head of Global Product Development. “The data we are presenting reinforce our ongoing commitment to redefining treatment paradigms, improving on existing standards of care and addressing a diversity of patient and healthcare system needs.”
Roche’s continued commitment to reinforcing strength of current portfolio
With 25 years of expertise in blood diseases, Roche has developed new medicines that changed the standard of care in several blood disorders with high unmet need. The data at this year’s meeting exemplify Roche’s commitment to investing in its current portfolio to further improve patient outcomes.
Interim data from the phase III HAVEN 7 study reinforce the efficacy and safety of Hemlibra® (emicizumab) in infants with severe haemophilia A without factor VIII inhibitors.1 For this population, early prophylaxis may prevent long-term damage to joints and muscles and potentially reduce the risk of intracranial haemorrhage, which can be life-threatening.
New data evaluating Polivy® (polatuzumab vedotin) that underscore the potential impact of this treatment for the diffuse large B-cell lymphoma (DLBCL) patient community will be shared at the meeting. Health-related quality of life (HRQoL) data from the phase III POLARIX study will be presented, highlighting the potential impact of Polivy in combination with MabThera®/Rituxan® (rituximab), cyclophosphamide, doxorubicin and prednisone (R-CHP) on reducing the need for subsequent treatments in people with previously untreated DLBCL, a population where multiple subsequent treatments can be a significant treatment burden.2 Based on data from the POLARIX study, this Polivy combination has been approved in the EU and recently, Japan, for the treatment of adult patients with previously untreated DLBCL.
Roche is presenting updated data from the broadest and most comprehensive CD20xCD3 bispecific antibody development programme in the industry. This aims to provide off-the-shelf, fixed-duration treatment options, which address the unique and diverse needs of people with blood cancers. Data include updated analyses for Lunsumio® (mosunetuzumab), the first CD20xCD3 T-cell engaging bispecific antibody approved by the European Commission to treat follicular lymphoma (FL), and glofitamab, for which data have been submitted for approval to the European Medicines Agency, and submissions to additional health authorities worldwide, including the U.S. Food and Drug Administration (FDA), are ongoing.
An updated analysis from the pivotal phase II GO29781 study of Lunsumio in people with relapsed or refractory (R/R) FL after two or more prior therapies will show continued durable responses across multiple key efficacy endpoints in addition to offering the potential to be administered in an outpatient setting.3 In addition, studies evaluating Lunsumio as a monotherapy and in novel combinations for the treatment of DLBCL in earlier lines of treatment will be presented, highlighting the potential of Lunsumio in other settings.
Updated results from the phase II NP30179 study will show a fixed course of glofitamab monotherapy can deliver durable complete responses in people with heavily pre-treated aggressive lymphomas.6,7 Results from the pivotal R/R DLBCL cohort indicate patients can maintain durable responses following fixed-duration treatment with glofitamab, potentially allowing them to benefit from a treatment-free period
Exploring and innovating in new areas of unmet need
Roche is applying its scientific expertise to expand its haematology clinical development programme by exploring additional blood diseases and bringing innovations that address the various needs of patients in areas of high unmet need.
The first phase III clinical data for crovalimab from the COMMODORE 3 study in China, will be presented at ASH. These data demonstrate that crovalimab met the co-primary efficacy endpoints, suggesting that crovalimab is efficacious and well-tolerated in people with PNH, a rare and life-threatening blood condition, where healthy red blood cells are targeted and destroyed by the body’s complement system.8 There are currently no effective treatment options for PNH broadly available in China.
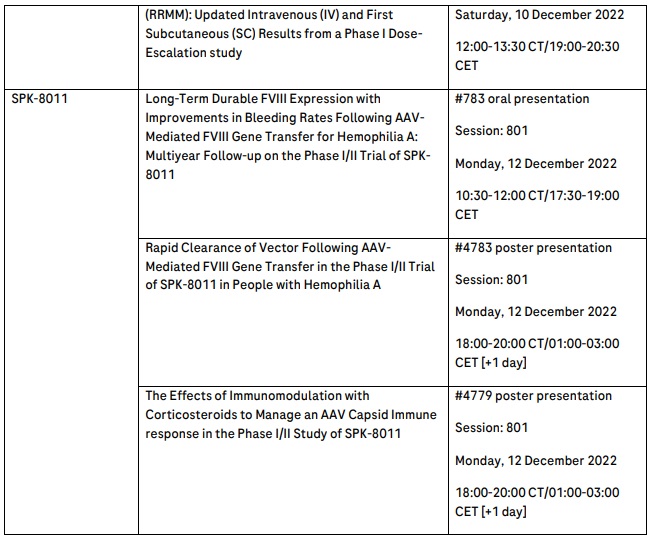
Spark Therapeutics, a member of the Roche Group, will share updated long-term follow-up data from the ongoing phase I/II clinical trial of SPK-8011, an investigational AAV-based gene therapy being developed for the treatment of haemophilia A.10 The acquisition of Spark Therapeutics brought new capabilities in haemophilia A to address the high unmet medical need for people living with this disease and endeavour to create additional benefit beyond current treatment options.
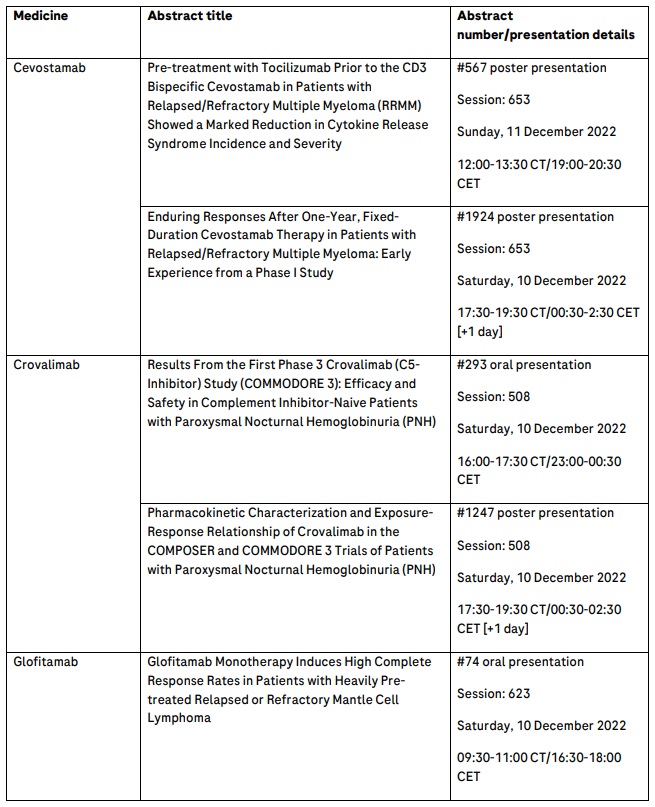
Positive data on cevostamab will be presented at ASH, including data from the phase I GO39775 study, which suggest that patients with heavily pre-treated multiple myeloma can maintain durable responses with fixed-duration cevostamab.11 Additionally, phase I data from Roche’s GPRC5DxCD3 T-cell engaging bispecific antibody, RG6234, showing encouraging preliminary activity in people with R/R multiple myeloma, will be presented.12 With this pipeline, Roche is committed to advancing treatments for multiple myeloma, which remains an incurable disease characterised by multiple relapses.
Further information on the key abstracts featuring Roche medicines that will be presented at ASH can be found in the table below.
Follow Roche on Twitter via @Roche and on LinkedIn and keep up to date with ASH Annual Meeting news and updates by using the hashtag #ASH22.
About Roche in haematology
Roche has been developing medicines for people with malignant and non-malignant blood diseases for more than 20 years; our experience and knowledge in this therapeutic area runs deep. Today, we are investing more than ever in our effort to bring innovative treatment options to patients across a wide range of haematologic diseases. Our approved medicines include MabThera®/Rituxan® (rituximab), Gazyva®/Gazyvaro® (obinutuzumab), Polivy® (polatuzumab vedotin), Venclexta®/Venclyxto® (venetoclax) in collaboration with AbbVie, Hemlibra® (emicizumab) and Lunsumio® (mosunetuzumab). Our pipeline of investigational haematology medicines includes T-cell engaging bispecific antibodies glofitamab, targeting both CD20 and CD3 and cevostamab, targeting both FcRH5 and CD3, Tecentriq® (atezolizumab), a monoclonal antibody designed to bind with PD-L1, and crovalimab, an anti-C5 antibody engineered to optimise complement inhibition. Our scientific expertise, combined with the breadth of our portfolio and pipeline, also provides a unique opportunity to develop combination regimens that aim to improve the lives of patients even further.
About Roche and Spark Therapeutics gene therapy research in haemophilia A
We believe gene therapy has the potential to revolutionise medicine and improve the lives of patients with genetic and other serious diseases. Pairing Roche’s long-standing commitment to developing medicines in haemophilia with Spark Therapeutics’ proven gene therapy expertise brings together the best team of collaborators researching gene therapies in haemophilia A.
It is our aligned objective to develop gene therapies for haemophilia A that, with the lowest effective dose and the optimal immunomodulatory regimen, demonstrate safety, predictability, efficacy, and durability for patients.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan