
Late-breaking results from Phase III trial of OCREVUS (ocrelizumab) subcutaneous injection and Phase II trial of BTK inhibitor fenebrutinib in multiple sclerosis (MS) will be presented
10-year OCREVUS efficacy and safety data show significant benefit in slowing long-term disability progression and consistent long-term safety profile in MS
Additional OCREVUS real-world and clinical data show impact for underrepresented populations including more than 3,200 pregnant women and Black and Hispanic/Latinx patients with MS
Longer-term safety data and late-breaking efficacy data from Phase III trial of ENSPRYNG (satralizumab) in neuromyelitis optica spectrum disorder (NMOSD) will be presented
Basel, 02 October 2023 - Roche (SIX: RO, ROG; OTCQX: RHHBY) will present new data for OCREVUS® (ocrelizumab) and investigational Bruton’s tyrosine kinase (BTK) inhibitor fenebrutinib for multiple sclerosis (MS), and ENSPRYNG® (satralizumab) for neuromyelitis optica spectrum disorder (NMOSD). In total, Roche will be presenting 36 abstracts at the 9th Joint ECTRIMS-ACTRIMS Meeting (European and Americas Committees for Treatment and Research in Multiple Sclerosis) from 11-13 October 2023. Late-breaking data in MS includes the Phase Ib OCARINA I and Phase III OCARINA II studies evaluating an investigational subcutaneous OCREVUS injection. In addition, the Phase II FENopta study of fenebrutinib for people living with MS and late-breaking ENSPRYNG data for people with NMOSD, which includes longer-term data from the Phase III SAkuraMoon study, will also be presented.
“It is gratifying to see that OCREVUS and ENSPRYNG continue to show a favourable benefit/risk profile over many years in MS and NMOSD, and we are also pleased to share late-breaking results from our investigational MS medicine fenebrutinib and OCREVUS subcutaneous injection,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “We’ve developed these latest innovations with the goal of further improving the day-to-day lives of those living with MS.”
Multiple sclerosis (MS)
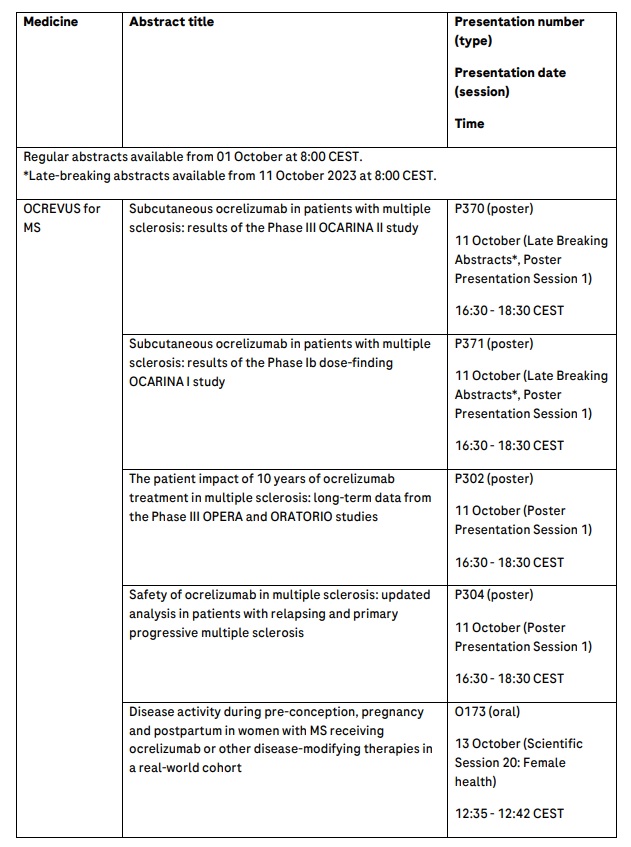
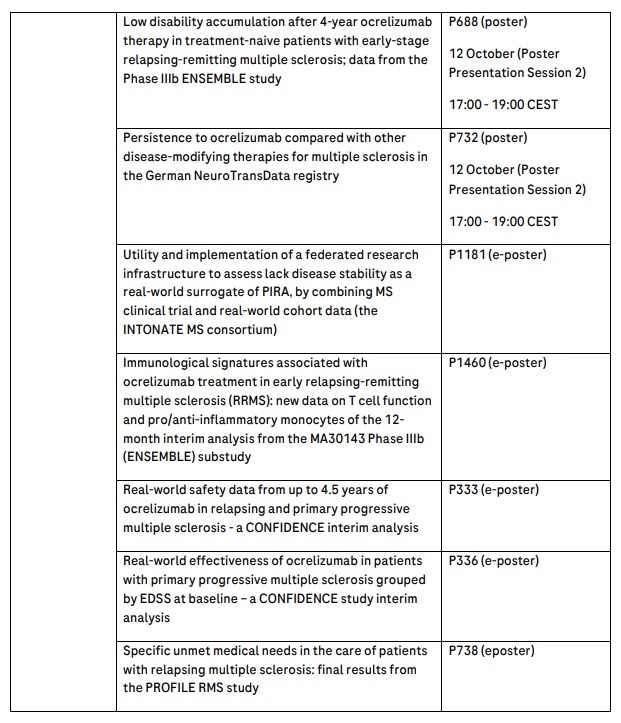
Roche will present 29 abstracts in MS, including three late-breaking presentations from the Phase Ib OCARINA I and Phase III OCARINA II studies on the OCREVUS subcutaneous injection in people with MS and the Phase II FENopta study of BTK inhibitor fenebrutinib in people with MS.
Highlights also include 10-year milestone data from the open-label extensions of Phase III OPERA I and II studies in relapsing MS (RMS) and ORATORIO study in primary progressive MS (PPMS) that show benefit on slowing long-term disability progression. OCREVUS is the only medicine approved for both RMS and PPMS, and by slowing disability progression it has fundamentally changed the landscape of MS treatment, with more than 300,000 patients treated globally. Safety outcomes from more than 6,000 patients across 12 OCREVUS clinical trials further support the medicine’s consistent favourable safety profile over 10 years.
Roche safety data will report pregnancy and infant outcomes from more than 3,200 pregnancies, and separate real-world data on pregnant women in the international MSBase registry will provide insights on the impact of OCREVUS and other disease-modifying therapies on relapses during and post-pregnancy. Further, one-year data from the first-ever clinical trial in Black and Hispanic/Latinx people with MS (Phase IV CHIMES trial) will show OCREVUS effectively controlled disease activity.
Neuromyelitis optica spectrum disorder (NMOSD)
Roche will present seven NMOSD abstracts, including late-breaking, longer-term data from the Phase III SAkuraMoon open-label extension study and real-world data evaluating ENSPRYNG in people with NMOSD.
Infection is a major comorbidity in people with NMOSD, and analyses comparing infection rates across clinical trials, post-marketing settings and U.S. claims data suggest overall lower rates in the ENSPRYNG-treated population.
Follow Roche on X via @Roche and keep up to date with ECTRIMS-ACTRIMS 2023 news and updates by using the hashtag #MSMilan2023. Below are the details of all Roche presentations.



About multiple sclerosis
Multiple sclerosis (MS) is a chronic disease that affects more than 2.8 million people worldwide. MS occurs when the immune system abnormally attacks the insulation and support around nerve cells (myelin sheath) in the central nervous system (brain, spinal cord and optic nerves), causing inflammation and consequent damage. This damage can cause a wide range of symptoms, including muscle weakness, fatigue and difficulty seeing, and may eventually lead to disability. Most people with MS experience their first symptom between 20 and 40 years of age, making the disease the leading cause of non-traumatic disability in younger adults.
People with all forms of MS experience disease progression – permanent loss of nerve cells in the central nervous system – from the beginning of their disease even if their clinical symptoms aren’t apparent or don’t appear to be getting worse. Delays in diagnosis and treatment can negatively impact people with MS, in terms of their physical and mental health, and contribute to the negative financial impact on the individual and society. An important goal of treating MS is to slow, stop and ideally prevent disease activity and progression as early as possible.
Relapsing-remitting MS (RRMS) is the most common form of the disease and is characterised by episodes of new or worsening signs or symptoms (relapses) followed by periods of recovery. Approximately 85% of people with MS are initially diagnosed with RRMS. The majority of people who are diagnosed with RRMS will eventually transition to secondary progressive MS (SPMS), in which they experience steadily worsening disability over time. Relapsing forms of MS (RMS) include people with RRMS and people with SPMS who continue to experience relapses. Primary progressive MS (PPMS) is a debilitating form of the disease marked by steadily worsening symptoms but typically without distinct relapses or periods of remission. Approximately 15% of people with MS are diagnosed with the primary progressive form of the disease. Until the FDA approval of OCREVUS, there had been no FDA-approved treatments for PPMS.
About OCREVUS (ocrelizumab)
OCREVUS is the first and only therapy approved for both RMS (including RRMS and active, or relapsing SPMS, in addition to clinically isolated syndrome [CIS] in the U.S.) and PPMS. OCREVUS is a humanised monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to myelin (nerve cell insulation and support) and axonal (nerve cell) damage. This nerve cell damage can lead to disability in people with MS. Based on preclinical studies, OCREVUS binds to CD20 cell surface proteins expressed on certain B cells, but not on stem cells or plasma cells, suggesting that important functions of the immune system may be preserved. OCREVUS is administered by intravenous infusion every six months. The initial dose is given as two 300 mg infusions given two weeks apart. Subsequent doses are given as single 600 mg infusions.
About fenebrutinib
Fenebrutinib is an investigational oral, reversible and non-covalent Bruton’s tyrosine kinase (BTK) inhibitor that blocks the function of BTK. BTK, also known as tyrosine-protein kinase BTK, is an enzyme that regulates B-cell development and activation and is also involved in the activation of innate immune system myeloid lineage cells, such as macrophages and microglia. Pre-clinical data have shown fenebrutinib to be potent and highly selective, and it is the only reversible inhibitor currently in Phase III trials for MS. Fenebrutinib has been shown to be 130 times more selective for BTK vs. other kinases. These design features may be important as the high selectivity and reversibility can potentially reduce off-target effects of a molecule.
Fenebrutinib is a dual inhibitor of both B-cell and microglia activation. This dual inhibition may be able to reduce both MS disease activity and progression, thereby potentially addressing the key unmet medical need in people living with MS. The Phase III programme includes two identical trials in RMS (FENhance 1 & 2) with an active teriflunomide comparator and one trial in primary progressive MS (PPMS) (FENtrepid) in which fenebrutinib is being evaluated against OCREVUS® (ocrelizumab). To date, more than 2,500 patients and healthy volunteers have been treated with fenebrutinib in Phase I, II and III clinical programmes across multiple diseases, including MS and other autoimmune disorders.
About ENSPRYNG (satralizumab)
ENSPRYNG, which was designed by Chugai, a member of the Roche Group, is a humanised monoclonal antibody that targets interleukin-6 (IL-6) receptor activity. ENSPRYNG was designed using novel recycling antibody technology which, compared to conventional technology, allows for longer duration of the antibody and subcutaneous dosing every four weeks.
Positive Phase III results for ENSPRYNG, as both monotherapy and in combination with baseline immunosuppressive therapy, demonstrate that IL-6 inhibition is an effective therapeutic approach for neuromyelitis optica spectrum disorder (NMOSD). ENSPRYNG is currently approved for NMOSD in 85 countries with further applications under review with numerous regulators. Roche continues to investigate ENSPRYNG in other autoantibody-mediated rare neurological diseases characterised by elevated IL-6 levels, indications including generalised Myasthenia Gravis (gMG), Myelin Oligodendrocyte Glycoprotein Antibody-associated Disease (MOGAD) and Autoimmune Encephalitis (AIE).
ENSPRYNG was granted Breakthrough Therapy Designation for the treatment of NMOSD by the FDA in December 2018 and designated as an orphan drug for NMOSD in the United States, Europe, Russia and Japan.
In addition,the FDA has designated satralizumab as an investigational orphan drug for gMG, MOGAD and AIE (NMDAR).
About Roche in Neuroscience
Neuroscience is a major focus of research and development at Roche. Our goal is to pursue ground-breaking science to develop new treatments that help improve the lives of people with chronic and potentially devastating diseases. Roche and Genentech are investigating more than a dozen medicines for neurological disorders, including MS, spinal muscular atrophy, neuromyelitis optica spectrum disorder, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, acute ischemic stroke, Duchenne muscular dystrophy and Angelman syndrome. Together with our partners, we are committed to pushing the boundaries of scientific understanding to solve some of the most difficult challenges in neuroscience today.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. More than thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Moreover, for the twelfth consecutive year, Roche has been recognised as one of the most sustainable companies in the Pharmaceuticals Industry by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020, Roche invested CHF 12.2 billion in R&D and posted sales of CHF 58.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan